- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
AQA A Level Chemistry復習筆記1.7.6 Catalysts
Maxwell-Boltzmann Distribution Curve - Catalyst
- Catalysis?is the process in which the rate of a chemical reaction is increased, by adding a?catalyst
- A catalyst increases the rate of a reaction by providing the reactants with an?alternative reaction pathway?which is?lower in activation energy?than the uncatalysed reaction
- Catalysts can be divided into two types:
- Homogeneous catalysts
- Heterogeneous catalysts
- Homogeneous?means that the catalyst is in the?same phase?as the reactants
- For example, the reactants and the catalysts are all in solution
- Heterogeneous?means that the catalyst is in a?different phase?to the reactants
- For example, the reactants are gases but the catalyst used is a solid
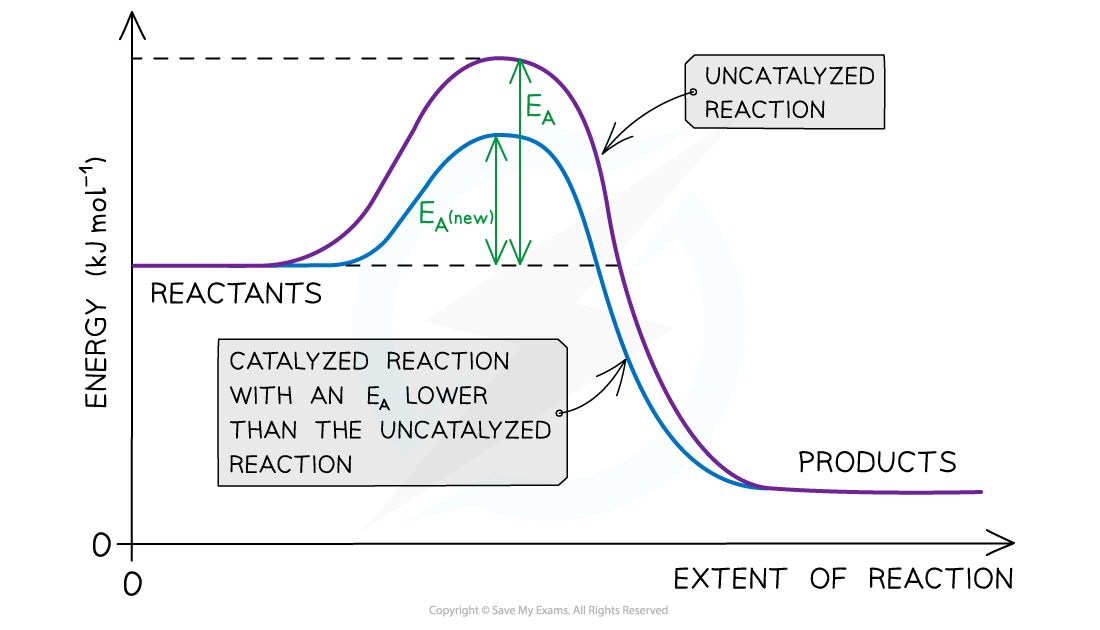
The diagram shows that the catalyst allows the reaction to take place through a different mechanism, which has a lower activation energy than the original reaction
Maxwell-Boltzmann distribution curve
- Catalysts?provide the reactants another pathway which has a lower activation energy
- By lowering?Ea,?a?greater proportion?of molecules in the reaction mixture have the activation energy, and therefore have sufficient energy for an?effective collision
- As a result of this, the rate of the catalysed reaction is increased compared to the uncatalyzed reaction
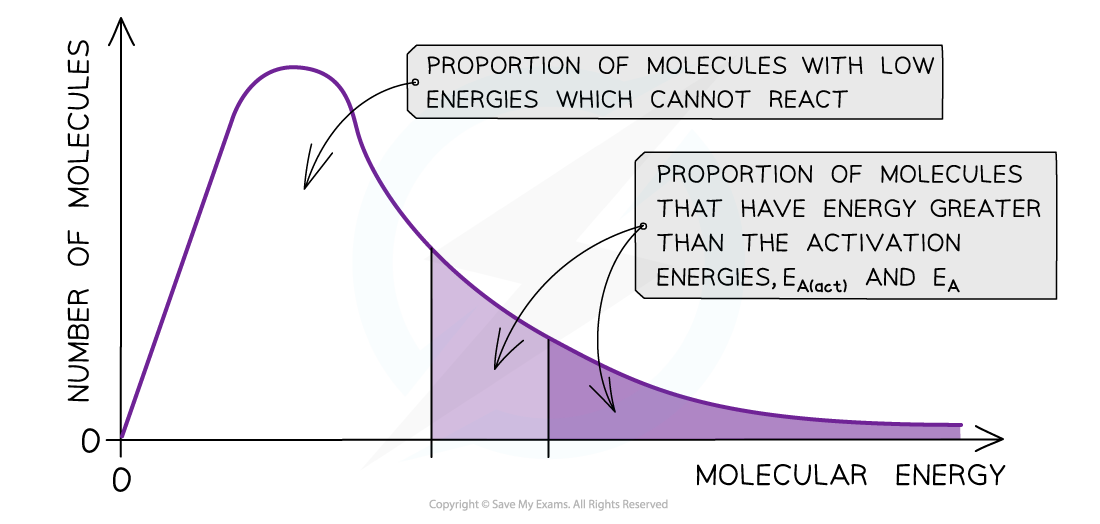
The diagram shows that the total shaded area (both dark and light shading) under the curve shows the number of particles with energy greater than the Eawhen a catalyst is present. This area is much larger than the dark shaded area which shows the number of particles with energy greater than the?Eawithout a catalyst
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









