- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
2015 AP Chemistry化學真題系列之選擇題免費下載
歷年AP Calculus BC微積分BC系列
真題與答案下載
真題下載請前往【純真題】小程序

翰林國際教育全網首發
力爭超快速發布最全資料
助你在升學路上一帆風順
為你的未來保駕護航
2015 AP Chemistry Practice Exam Multiple Choice Free Download
2015 AP 化學模考選擇題部分免費下載
此套Section I試卷共分計時1小時30分鐘組成
共50題
僅限使用鉛筆,無計算器
考試時會提供花常用的等式與常量
以及化學元素周期表
完整版下載鏈接見文末
部分真題預覽:
1)A 0.5mol sample of He(g) and a 0.5 mol sample of Ne(g) are placed separately in two 10.0L rigid containers at 25°C. Each container has a pinhole opening. Which of the gases, He(g) or Ne(g), will escape faster through the pinhole and why?
- He(g) will escape faster because the He(g) atoms are moving at a higher?average speed than the Ne(g) atoms.
- Ne(g) will escape faster because its initial pressure in the container is higher.
- Ne(g) will escape faster because the Ne(g) atoms have a higher average kinetic energy than the He(g) atoms.
- Both gases will escape at the same rate because the atoms of both gases have the same average kinetic energy.
2)The lattice energy of a salt is related to the energy required to separate the ions. For which of the following pairs of ions is the energy that is required to separate the ions largest? (Assume that the distance between the ions in each pair is equal to the sum of the ionic radii.)
- Na+(g) and Cl-(g)
- Cs+(g) and Br-(g)
- Mg2+(g) and O2-(g)
- Ca2+(g) and O2-(g)
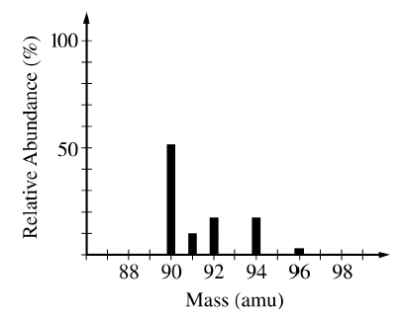
3)The mass spectrum of element X is presented in the diagram above. Based on the spectrum, which of the following can be concluded about element X?
- X is a transition metal, and each peak represents an oxidation state of the metal.
- X contains five electron sublevels.
- The atomic mass of X is 90.
- The atomic mass of X is between 90 and 92.
4)Which of the following diagrams best depicts an alloy of Ni and B ?
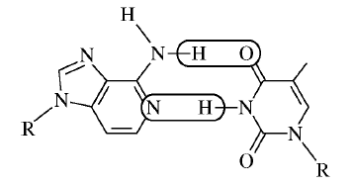
5) Which of the following is the strongest type of interaction that occurs between the atoms within the circled areas of the two molecules represented above?
- Polar covalent bond
- Nonpolar covalent bond
- Hydrogen bond
- London dispersion forces
6)A hot iron ball is dropped into a 200.g sample of water initially at 50.°C. If 8.4 kJ of heat is transferred from the ball to the water, what is the final temperature of the water? (The specific heat of water is 4.2J/(g.°C).)
- 40.°C
- 51.°C
- 60.°C
- 70.°C
完整版真題資料可以底部二維碼免費領取↓↓↓
[vc_btn title="查看更多AP Chemistry化學課程相關詳情" color="primary" align="center" i_icon_fontawesome="fa fa-globe" css_animation="zoomIn" button_block="true" add_icon="true" link="url:http%3A%2F%2Fwww.linstitute.net%2Farchives%2F25860||target:%20_blank|"]

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1













