- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Edexcel A Level Chemistry:復習筆記6.3.4 Ligand Exchange
Ligand Exchange
- Ligand exchange?(or?ligand?substitution) is when one?ligand?in a complex is replaced by another
- Ligand exchange forms a new complex that is?more stable?than the original one
- The ligands in the original complex can be?partially?or?entirely?substituted by others
- The complex ion can change its charge or remain the same depending on the ligand involved
- There are no changes in coordination number, or the geometry of the complex, if the ligands are of a?similar?size
- But, if the ligands are of a?different size, for example water ligands and chloride ligands, then a change in coordination number and the geometry of the complex will occur
- Addition of a high concentration of chloride ions (from conc HCl or saturated NaCl) to an aqueous ion leads to a ligand substitution reaction.
- The Cl-?ligand is?larger?than the uncharged H2O and NH3?ligands so therefore ligand exchange can involve a change of co-ordination number
- For example when concentrated hydrochloric acid is added slowly and continuously to a copper(II) sulfate solution the colour changes from blue to green then finally yellow
- The equation for this reaction is
[Cu(H2O)6]2+?(aq)?+ 4Cl-?(aq) ? [CuCl4]2-?(aq) + 6H2O (l)
- We can see that all six water ligands have been replaced by four chloride ions
- This reaction involves a change in coordination number from 6 to 4
- Note that despite the charge on the complex changing from +2 to -2, there has been no change in oxidation number of the copper
- We can also see that this reaction is reversible, which helps to explain the observed colour change
- The hexaaquacopper(II) ion is blue
- The tetrachlorocuprate(II) ion is yellow
- The green colour is due to a mixture of the blue and yellow complex ions
- A similar reaction also takes place with cobalt resulting in a blue solution and a change in coordination number from 6 to 4
[Co(H2O)6]2+?(aq)?+ 4Cl-?(aq) ? [CoCl4]2-?(aq) + 6H2O (l)
Exam Tip
Be careful: If solid copper chloride (or any other metal) is dissolved in water it forms the aqueous [Cu(H2O)6]2+?complex and not the chloride [CuCl4?]2-?complex
The Chelate Effect & Stability
- The replacement of monodentate ligands with bidentate and multidentate ligands in complex ions is called the?chelate effect
- It is an energetically favourable reaction, meaning that?ΔG??is negative
- The driving force behind the reaction is entropy
- The Gibbs equation reminds us of the link between enthalpy and entropy:
ΔG??= ΔHreaction??– TΔSsystem?
- Reactions in solution between aqueous ions usually come with relatively small enthalpy changes
- However, the entropy changes are always positive in chelation because the reactions produce a net increase in the number of particles
- A small enthalpy change and relative large positive entropy change generally ensures that the overall free energy change is negative
- For example, when EDTA chelates with aqueous cobalt(II) two reactants becomes seven product species
[Co(H2O)6?]2+?(aq)?+ EDTA4-?(aq) → [CoEDTA]2-?(aq) + 6H2O?(l)
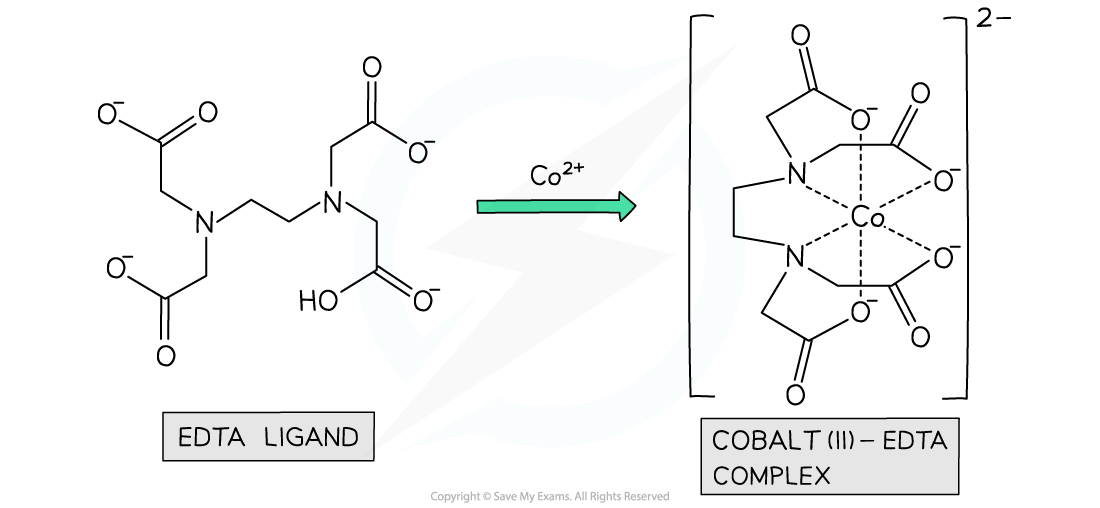
The ligand EDTA readily chelates with aqueous transition metal ions in an energetically favourable?reaction
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









