- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記2.1.5 Period 3 Chlorides
Reaction of Period 3 Chlorides & Water
- Chlorides of Period 3 elements show characteristic behaviour when added to water which can be explained by looking at their?chemical bonding?and?structure
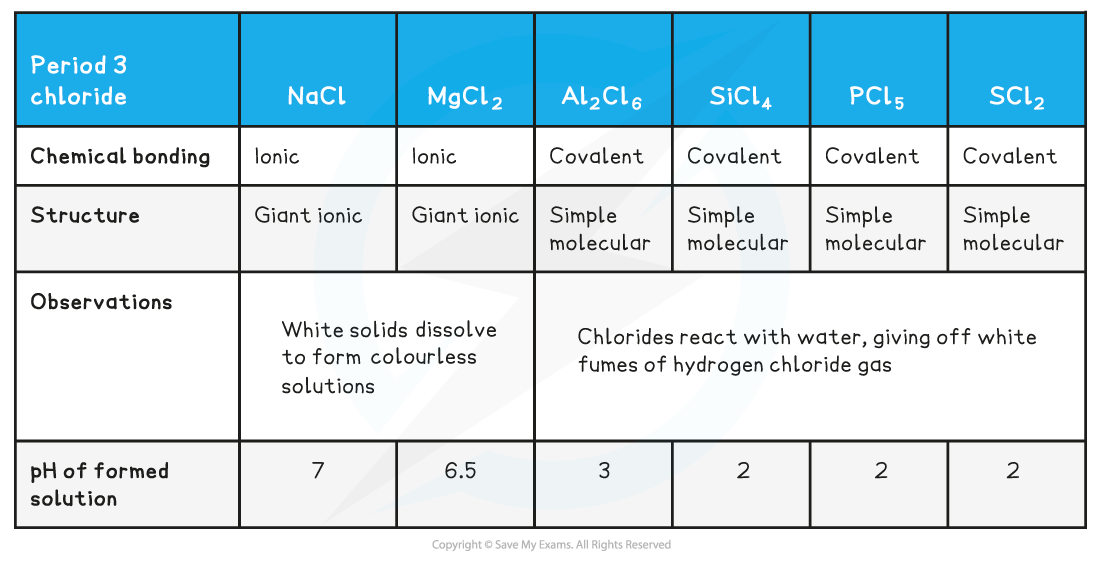
Chemical bonding & structure of Period 3 chlorides table
Sodium & magnesium chloride
- NaCl and MgCl2?do not react with water as the?polar?water molecules are attracted to the ions?dissolving?the chlorides and breaking down the?giant ionic structures: the metal and chloride ions become hydrated ions
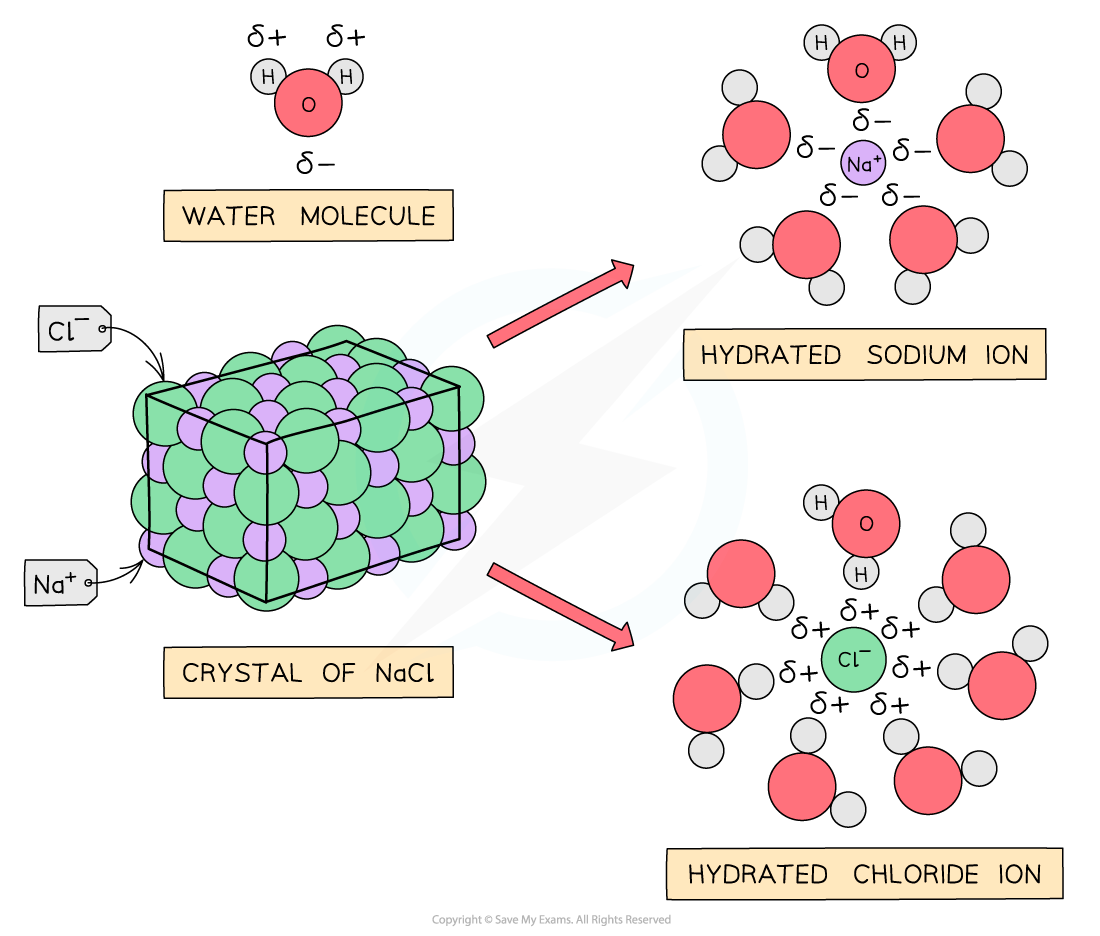 The diagram shows water molecules breaking down the giant ionic structure of NaCl and MgCl2?to form hydrated ions
The diagram shows water molecules breaking down the giant ionic structure of NaCl and MgCl2?to form hydrated ions
Aluminium chloride
- Aluminium chloride exists in two forms:
- AlCl3?as a?giant lattice?and with?ionic?bonds
- Al2Cl6?as a?dimer?with?covalent?bonds
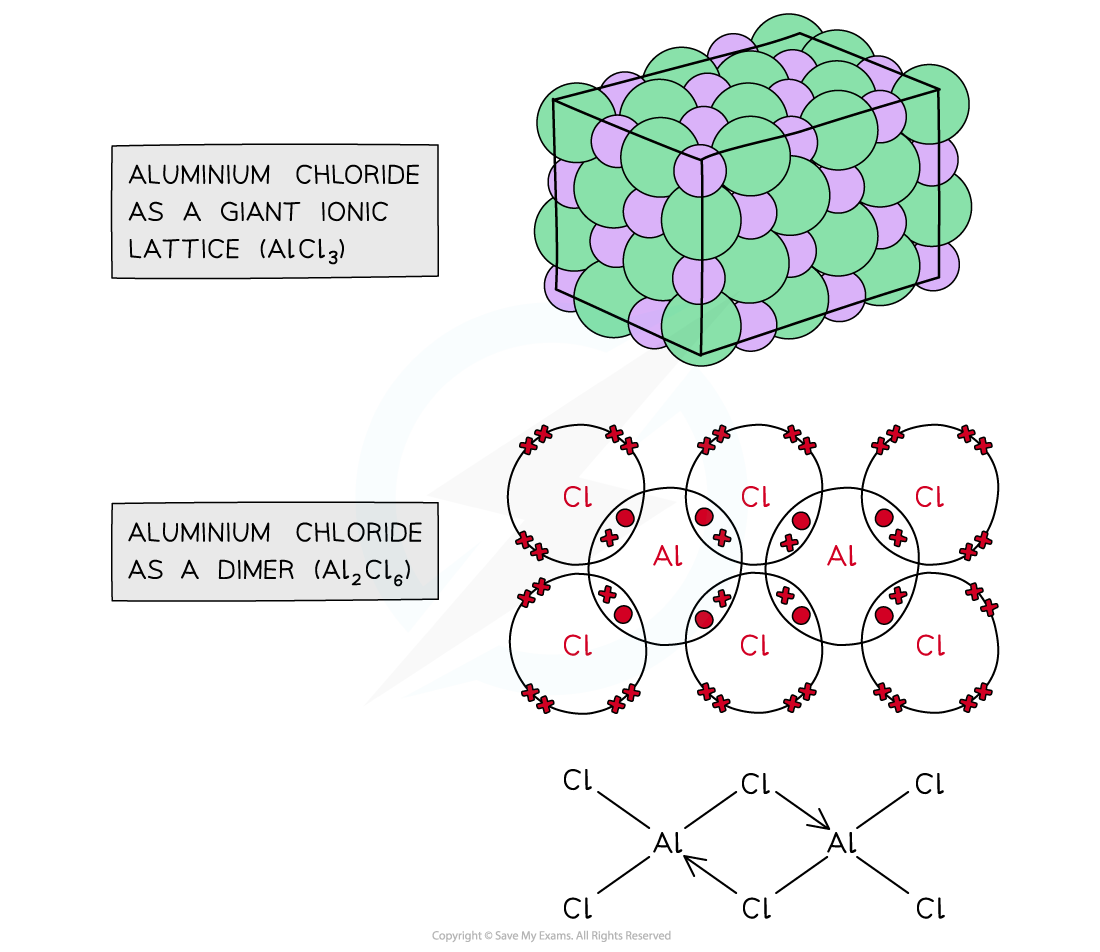
The two forms in which aluminium chloride exists
- When water is added to aluminium chloride the dimers are broken down and Al3+?and Cl-?ions enter the solution
- The highly charged Al3+?ion becomes hydrated and causes a water molecule that is bonded to the Al3+?to lose an H+?ion which turns the solution?acidic
- The H+?and the Cl-?form?hydrogen chloride gas?which is given off as?white fumes
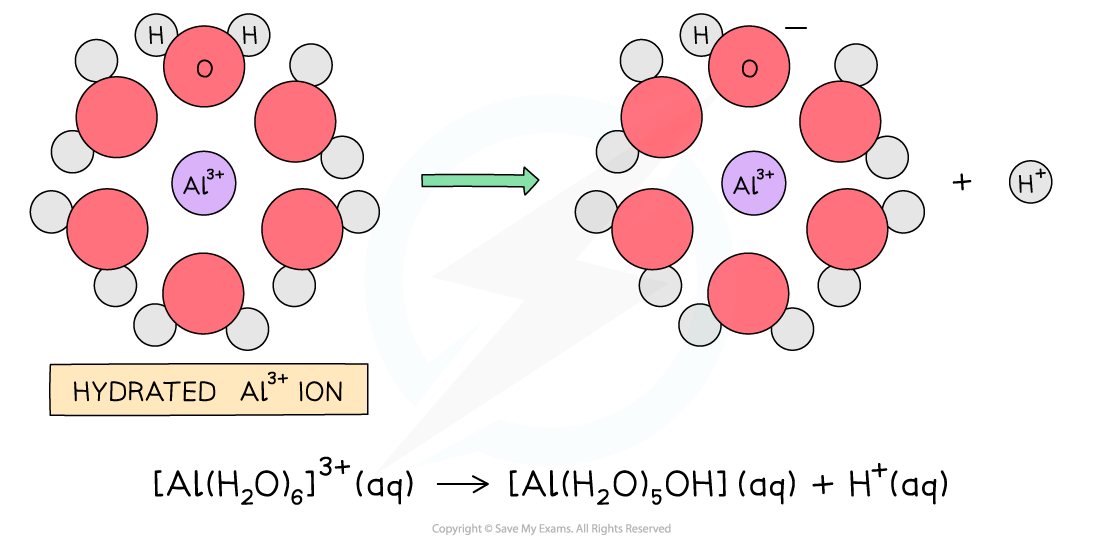
The hydrated aluminium causes a water molecule to lose a H+?ion turning the solution acidic
Silicon chloride
- SiCl4?is?hydrolysed?in water, releasing?white fumes of hydrogen chloride gas?in a?rapid?reaction
SiCl4(l) + 2H2O(l) → SiO2(s) + 4HCl(g)
- The SiO2?is seen as a?white precipitate?and some of the?hydrogen chloride gas?produced dissolves in water to form an?acidic?solution
Phosphorus(V) chloride
- PCl5?also gets?hydrolysed?in water
PCl5(s) + 4H2O(l) → H3PO4(aq) + 5HCl(g)
- Both H3PO4?and dissolved HCl are highly?acidic
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









