- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記1.7.13 Indicators used in Titration
Indicators
- Indicators?are substances that change colour when they are added to acidic or alkaline solutions
- When choosing the appropriate indicator, the pH of the equivalence point is very important
- The two most common indicators that are used in titrations are?methyl orange?and?phenolphthalein
- Both indicators change colour over a specific pH range
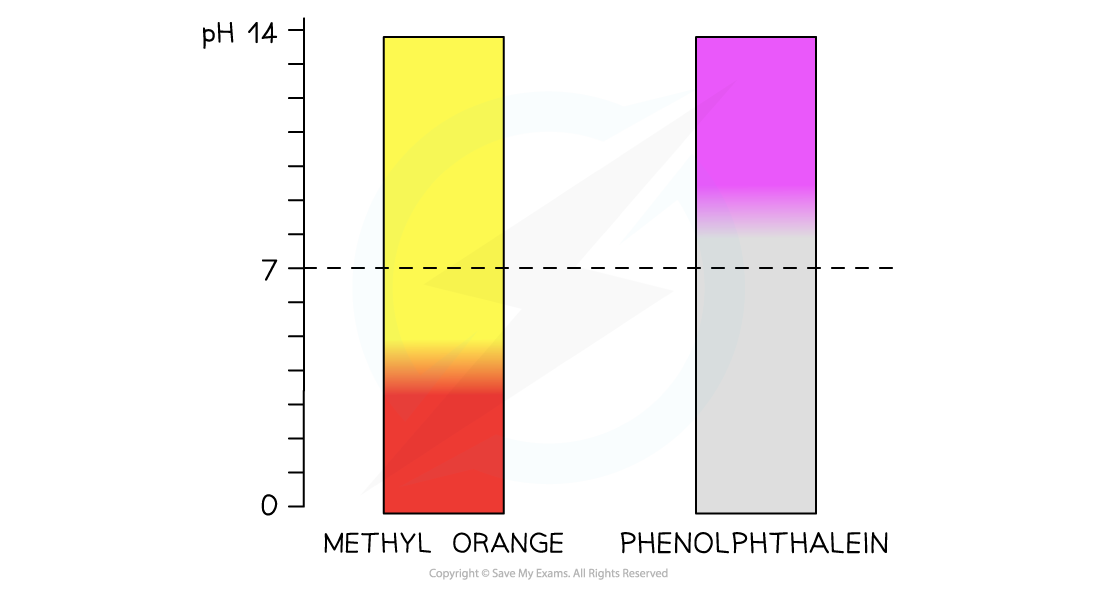
The diagram shows the change in colour from red to yellow of methyl orange over a pH range of 3.1-4.4 and from colourless to pink of phenolphthalein over a pH range of 8.3-10
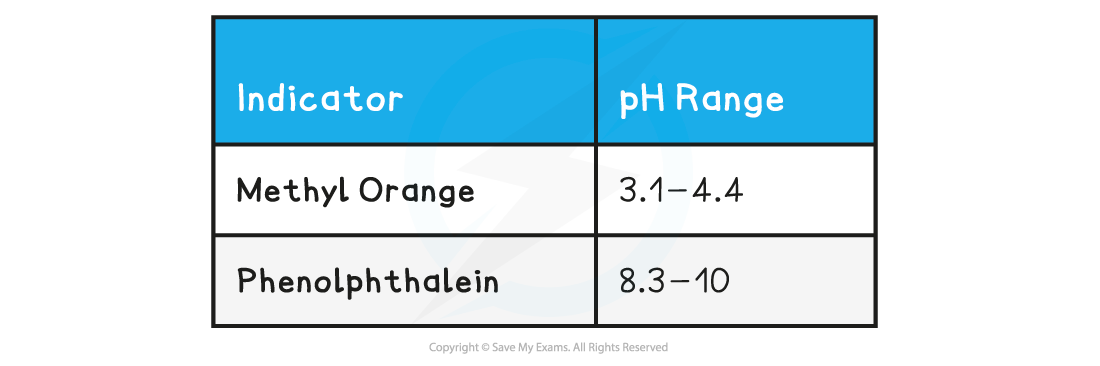
Indicator & pH range table
Choosing indicators for titrations
- Strong acid and strong alkali
- The colour change for?both indicators?takes place at a pH range that falls within the vertical region of the curve
- Therefore, either indicator can be used
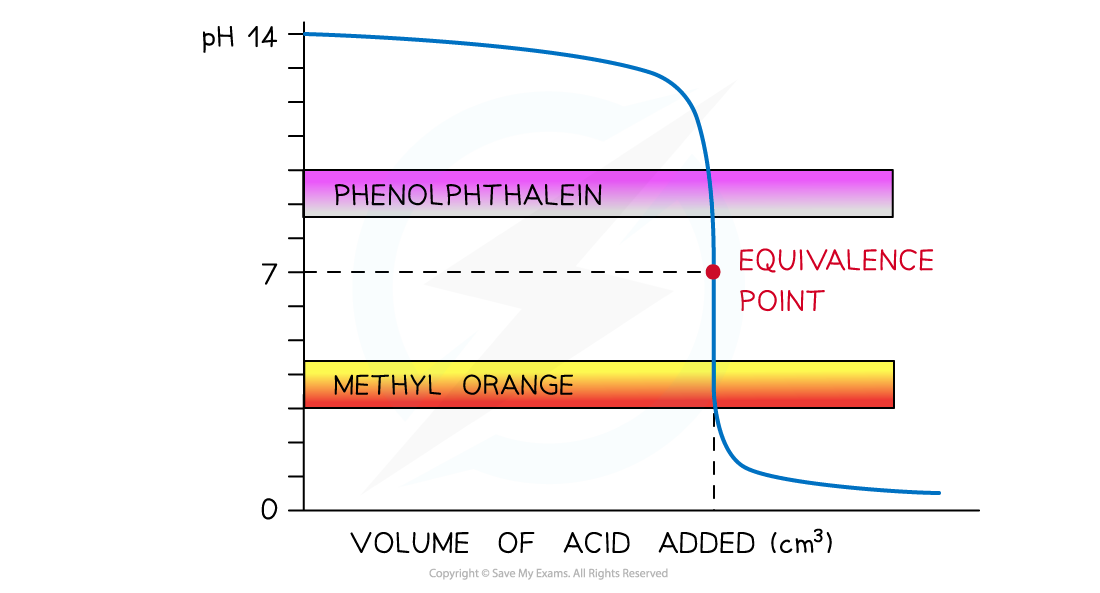
The diagram shows that both indicators can be used to determine the endpoint of the titration of a strong acid and strong alkali
- Strong acid and weak alkali
- Only?methyl orange?will change colour at a pH close to the equivalence point and within the vertical region of the curve
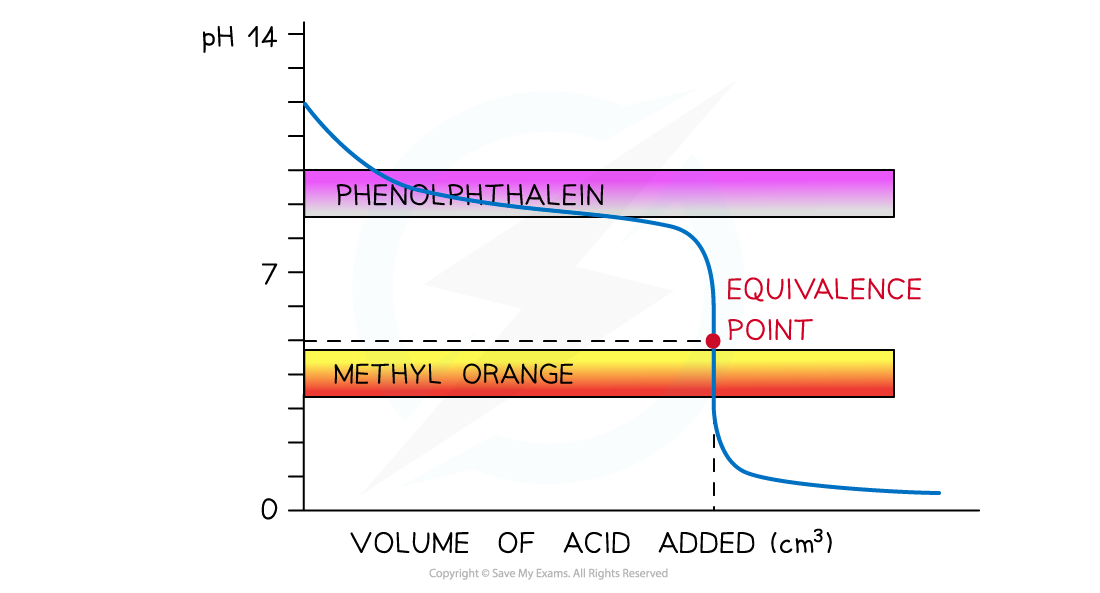
The diagram shows that only methyl orange can be used to determine the endpoint of the titration of a strong acid and weak alkali
- Weak acid and strong alkali
- Now, only?phenolphthalein?will change colour at a pH close to the equivalence point and within the vertical region of the curve
- The pH range at which methyl orange changes colour falls below the curve
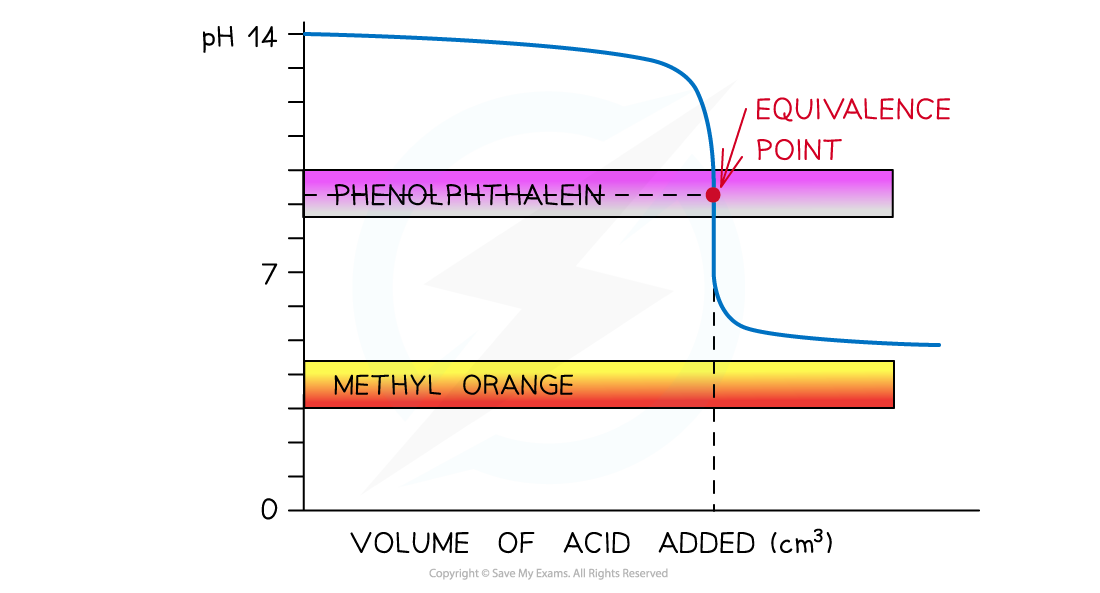 The diagram shows that only phenolphthalein can be used to determine the endpoint of the titration of a weak acid and strong alkali
The diagram shows that only phenolphthalein can be used to determine the endpoint of the titration of a weak acid and strong alkali
- Weak acid and weak alkali
- Neither indicator is useful, and a different method should be considered
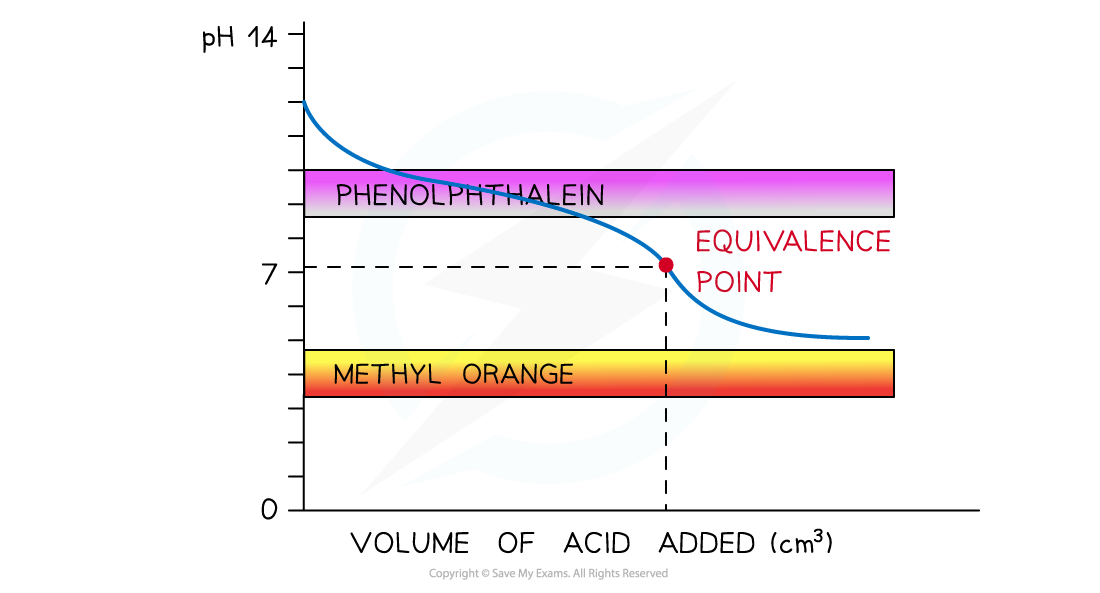
The diagram shows that nether indicators can be used to determine the endpoint of the titration of a weak acid and weak alkali
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









