- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記1.3.14 Van der Waals' Forces
van der Waals' Forces & Dipoles
- Covalent bonds are strong?intramolecular forces
- Molecules also contain weaker?intermolecular forces?which are forces?between?molecules
- These intermolecular forces are called?van der Waals’ forces
- There are two types of van der Waals’ forces:
- Instantaneous?(temporary)?dipole – induced dipole forces?also called?London dispersion forces
- Permanent dipole – permanent dipole forces
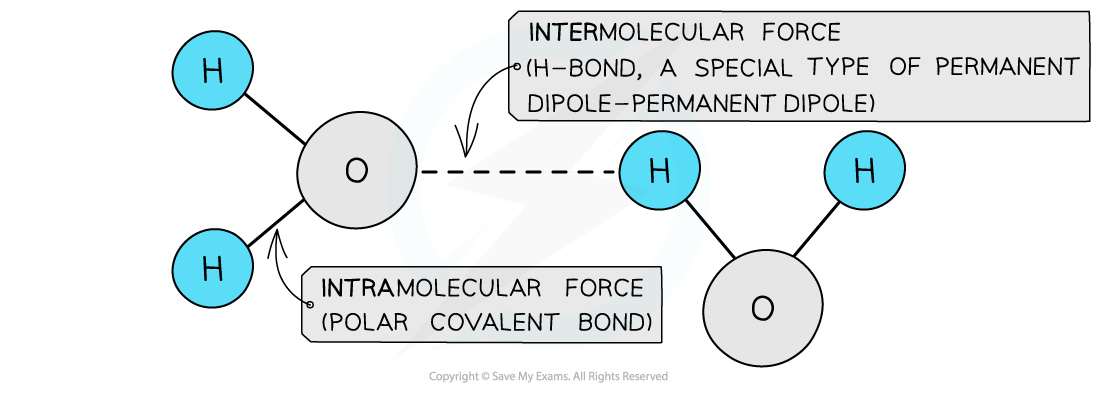
The polar covalent bonds between O and H atoms are intramolecular forces and the permanent dipole – permanent dipole forces between the molecules are intermolecular forces as they are a type of van der Waals’ force
Instantaneous dipole - induced dipole (id - id)
- Instantaneous dipole - induced dipole forces?or?London dispersion forces?exist between all atoms or molecules
- The?electron charge cloud?in non-polar molecules or atoms are constantly moving
- During this movement, the electron charge cloud can be more on one side of the atom or molecule than the other
- This causes a?temporary dipole?to arise
- This?temporary dipole?can?induce?a dipole on neighbouring molecules
- When this happens, the?δ+ end of the dipole?in one molecule and the?δ- end of the dipole?in a neighbouring molecule are?attracted?towards each other
- Because the electron clouds are moving constantly, the dipoles are only?temporary
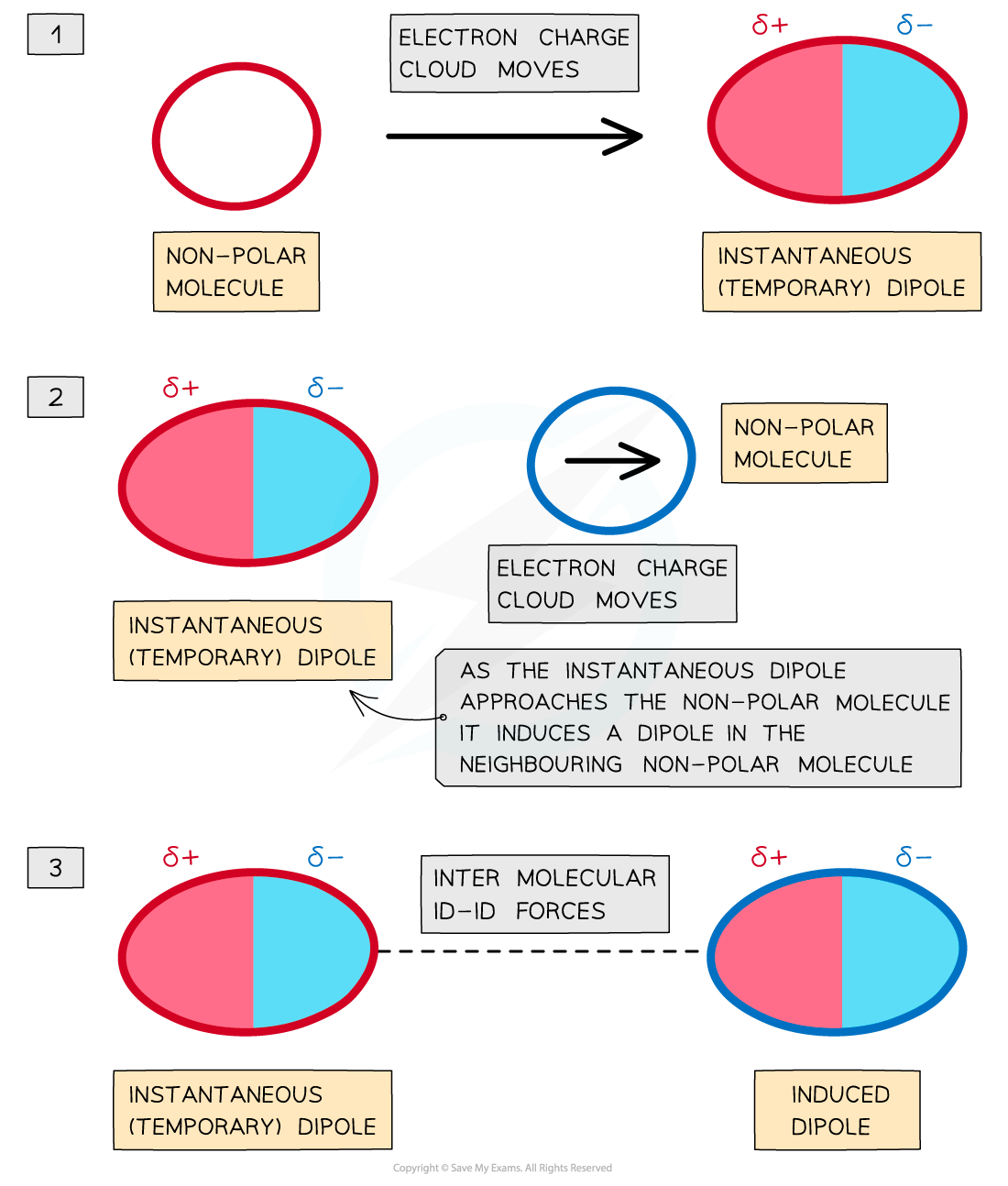
Id-id (London dispersion) forces between two non-polar molecules
- Id - id forces increase with:
- Increasing number of electrons (and?atomic number) in the molecule
- Increasing the places where the molecules come close together
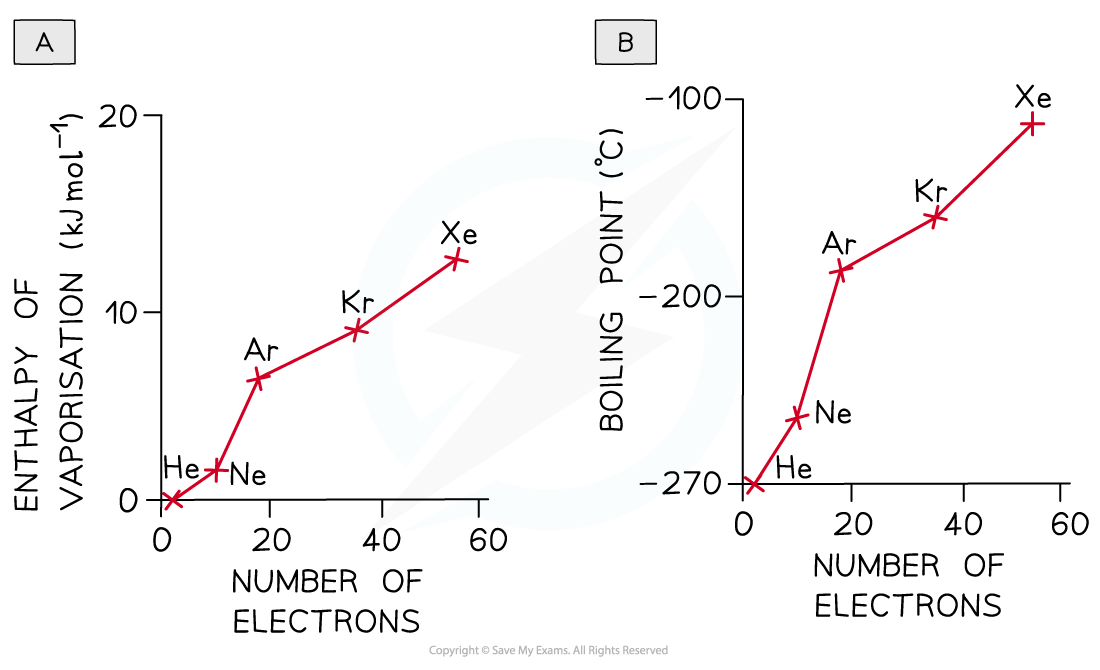 Going down the Group, the id-id forces increase due to the increased number of electrons in the atoms
Going down the Group, the id-id forces increase due to the increased number of electrons in the atoms
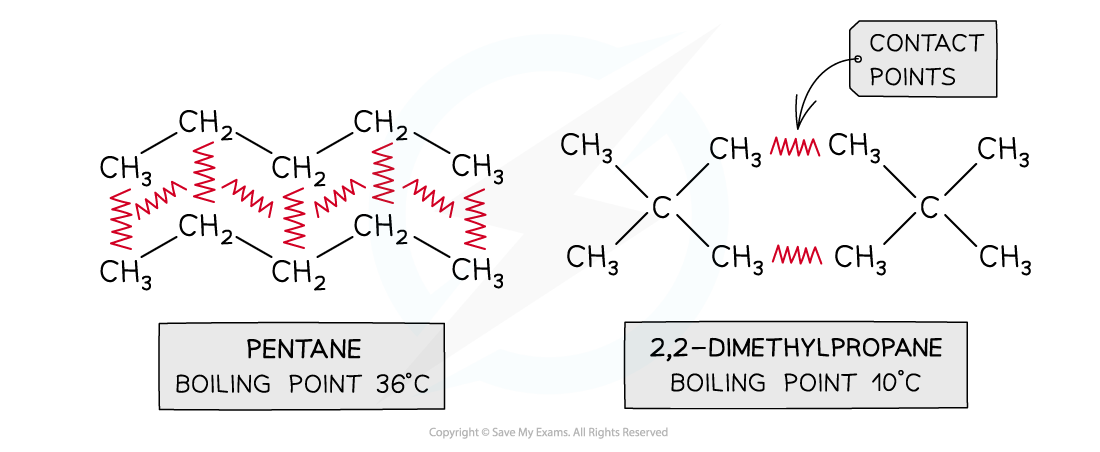
The increased number of contact points in petane means that it has more id-id forces and therefore a higher boiling point
Permanent dipole - permanent dipole (pd - pd)
- Polar molecules?have?permanent dipoles
- The molecule will always have a?negatively?and?positively charged end
- Forces between two molecules that have permanent dipoles are called?permanent dipole - permanent dipole forces
- The?δ+ end of the dipole?in one molecule and the?δ- end of the dipole?in a neighbouring molecule are?attracted?towards each other
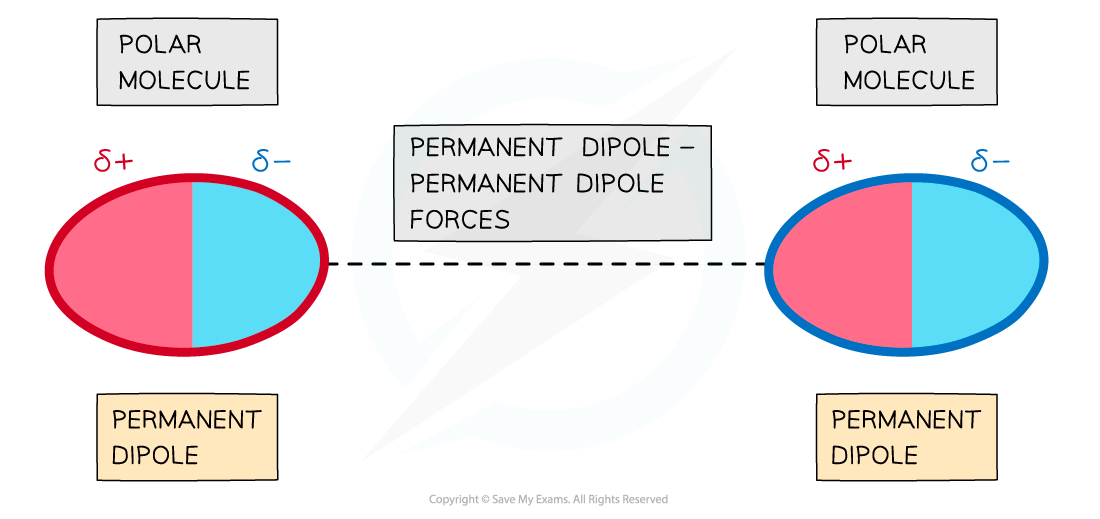
The delta negative end of one polar molecule will be attracted onwards the delta positive end of a neighbouring polar molecule
- For small molecules with?the same number of electrons,?pd - pd forces are?stronger?than id - id
- Butane and propanone have the same number of electrons
- Butane is a nonpolar molecule and will have id - id forces
- Propanone is a polar molecule and will have pd - pd forces
- Therefore, more energy is required to break the intermolecular forces between propanone molecules than between butane molecules
- So, propanone has a higher boiling point than butane
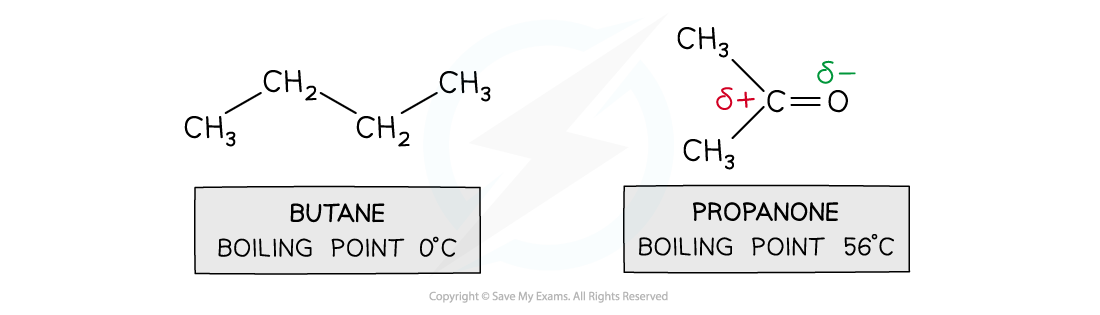
Pd-pd forces are stronger than id-id forces in smaller molecules with an equal number of electrons
Exam Tip
Remember this difference: intramolecular forces are forces?within?a molecule, whereas intermolecular forces are forces?between?a molecule.
Hydrogen Bonding as a Permanent Dipole
- Hydrogen bonding?is an?intermolecular force?between molecules with an -OH/-NH group and molecules with an N/O atom
- Hydrogen bonding is a special case of a?permanent dipole - dipole force?between molecules
- Hydrogen bonds are?stronger?forces than pd - pd forces
- The hydrogen is bonded to an O/N atom which is so?electronegative, that almost all the electron density from the covalent bond is drawn towards the O/N atom
- This leaves the H with a?large?delta positive?and the O/N with a?large delta negative charging?resulting in the formation of a?permanent dipole?in the molecule
- A?delta positive H?in one molecule is?electrostatically attracted?to the?delta negative?O/N?in a neighbouring molecule
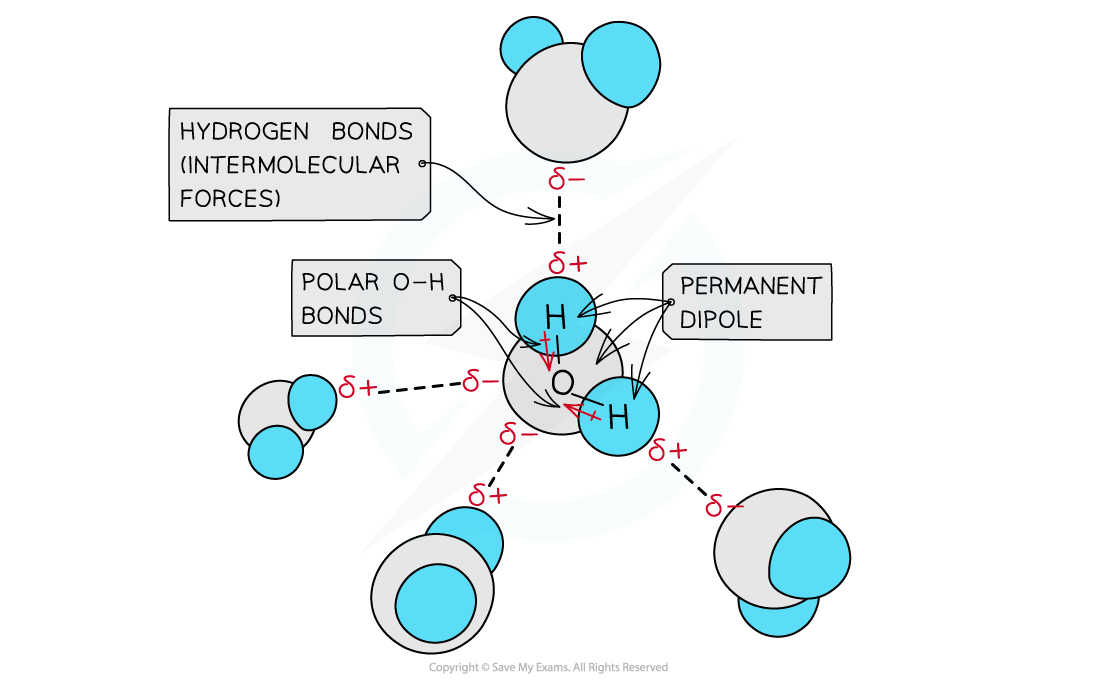
Hydrogen bonds in water molecules
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









