- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
AQA A Level Chemistry復習筆記5.1.8 Reaction Feasibility
Reaction Feasibility
- The Gibbs equation can be used to calculate whether a reaction is?feasible?or not
ΔG??= ΔHreaction??- TΔSsystem?
- When ΔG??is?negative, the reaction is?feasible?and likely to occur
- When ΔG?is?positive, the reaction is?not feasible?and unlikely to occur
- Feasible?and?spontaneous?are fairly similar terms to describe reactions
- Feasible tends to be used to describe reactions which are energetically favourable, so?reactions that should go
- Spontaneous tends to be used to describe?reactions that go of their own accord
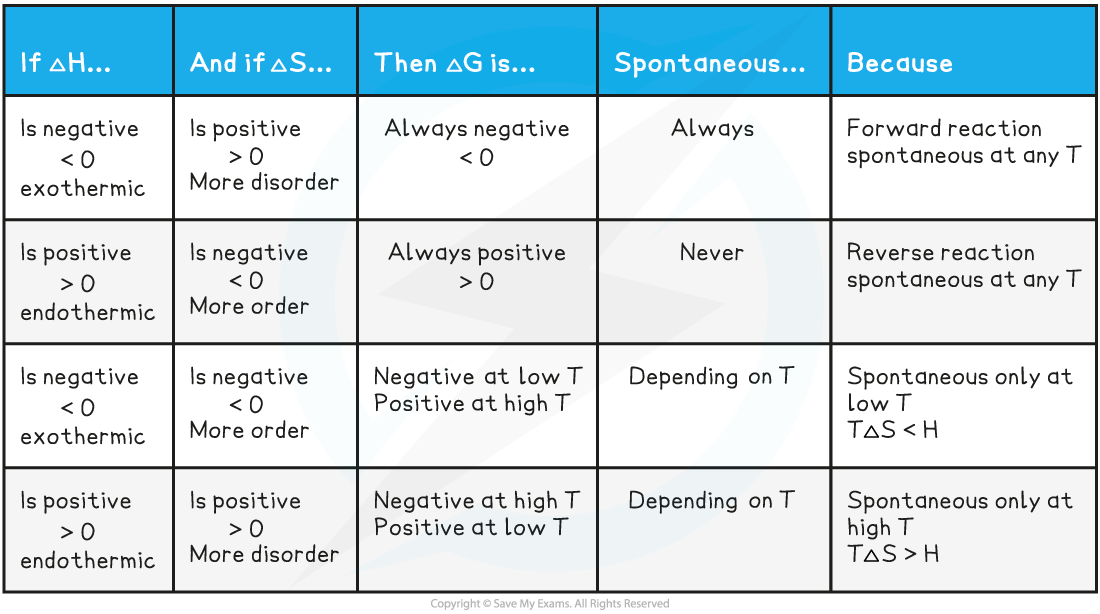
Summary for temperature and Gibbs free energy
Worked Example
Determining the feasibility of a reactionCalculate the Gibbs free energy change for the following reaction at 298 K and determine whether the reaction is feasible.
2Ca (s) + O2?(g) → 2CaO (s)? ? ? ? ?ΔH =?-635.5 kJ mol-1
S?[Ca(s)] = 41.00 J K-1?mol-1
S?[O2(g)] = 205.0 J K-1?mol-1
S?[CaO(s)] = 40.00 J K-1?mol-1
Answer
Step 1:?Calculate ΔSsystem?
ΔSsystem??= ΣΔSproducts??- ΣΔSreactants?
ΔSsystem??= (2 x ΔS??[CaO(s)]) -? (2 x ΔS??[Ca(s)] + ΔS??[O2(g)])
= (2 x 40.00) - (2 x 41.00 + 205.0)
= -207.0 J K-1?mol-1
Step 2:?Convert ΔS??to kJ K-1?mol-1
=?-207.0 J K-1?mol-1÷ 1000 = -0.207 kJ mol-1
Step 3:?Calculate ΔG?
ΔG??= ΔHreaction??- TΔSsystem?
= -635.5 - (298 x -0.207)
= -573.8 kJ mol-1
Step 4:?Determine whether the reaction is feasible
Since the ΔG??is?negative?the reaction is?feasible
Reaction Feasibility: Temperature Changes
- The?feasibility?of a reaction can be affected by the?temperature
- The Gibbs equation will be used to explain what will affect the feasibility of a reaction for exothermic and endothermic reactions

Exothermic reactions
- In exothermic reactions, ΔHreaction??is?negative
- If the ΔSsystem??is?positive:
- Both the first and second term will be?negative
- Resulting in a?negative?ΔG??so the reaction is?feasible
- Therefore, regardless of the temperature, an exothermic reaction with a positive ΔSsystem??will?always be feasible
- If the ΔSsystem??is?negative:
- The first term is?negative?and the second term is?positive
- At very high temperatures, the -TΔSsystem??will be very?large?and?positive?and will overcome ΔHreaction?
- Therefore, at high temperatures ΔG??is?positive?and the reaction is?not feasible
- Since the relative size of an entropy change is much smaller than an enthalpy change, it is unlikely that TΔS >?ΔH?as temperature increases
- These reactions are therefore usually spontaneous at normal conditions
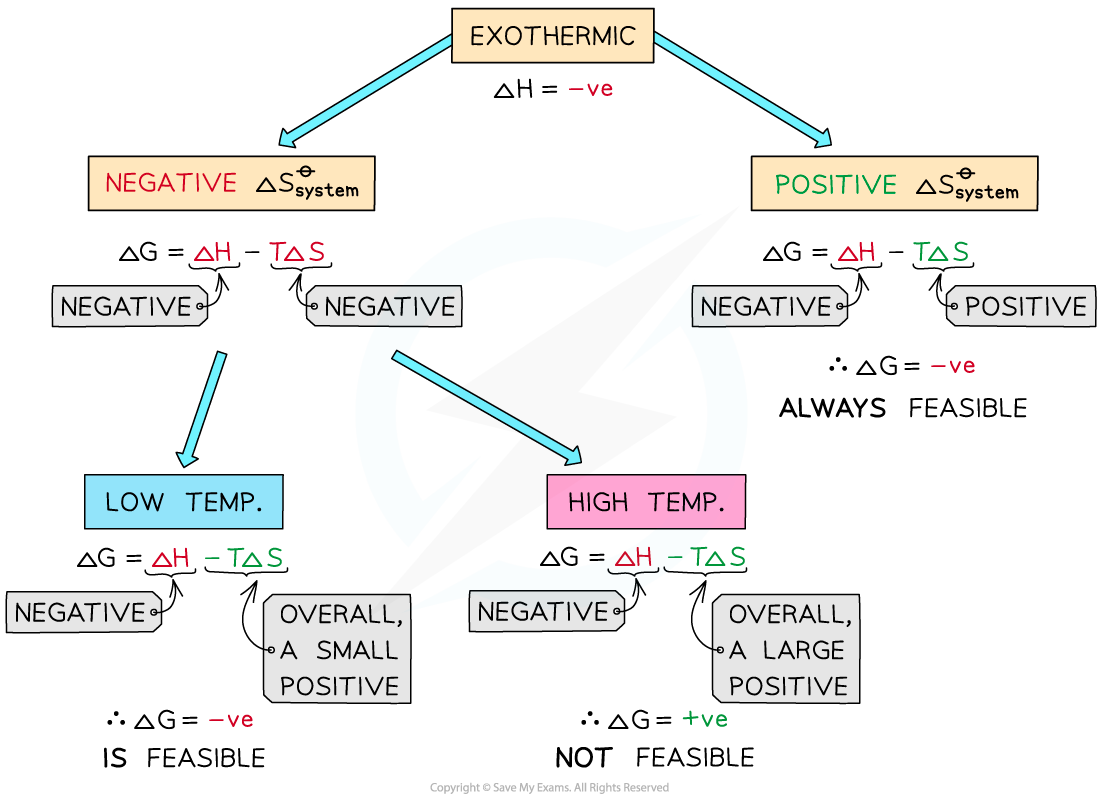
The diagram shows under which conditions exothermic reactions are feasible
Endothermic reactions
- In endothermic reactions, ΔHreaction??is?positive
- If the ΔSsystem??is?negative:
- Both the first and second term will be?positive
- Resulting in a?positive?ΔG??so the reaction is?not feasible
- Therefore, regardless of the temperature, endothermic with a negative ΔSsystem??will?never be feasible
- If the ΔSsystem??is?positive:
- The first term is?positive?and the second term is?negative
- At low temperatures, the -TΔSsystem??will be?small?and?negative?and will not overcome the larger ΔHreaction?
- Therefore, at low temperatures ΔG??is?positive?and the reaction is not feasible
- The reaction is?more?feasible?at?high temperatures?as the second term will become negative enough to overcome the ΔHreaction??resulting in a negative ΔG?
- This tells us that for certain reactions which are not feasible at room temperature, they can become feasible at higher temperatures
- An example of this is found in metal extractions, such as the extraction if iron in the blast furnace, which will be unsuccessful at low temperatures but can occur at higher temperatures (~1500?oC in the case of iron)
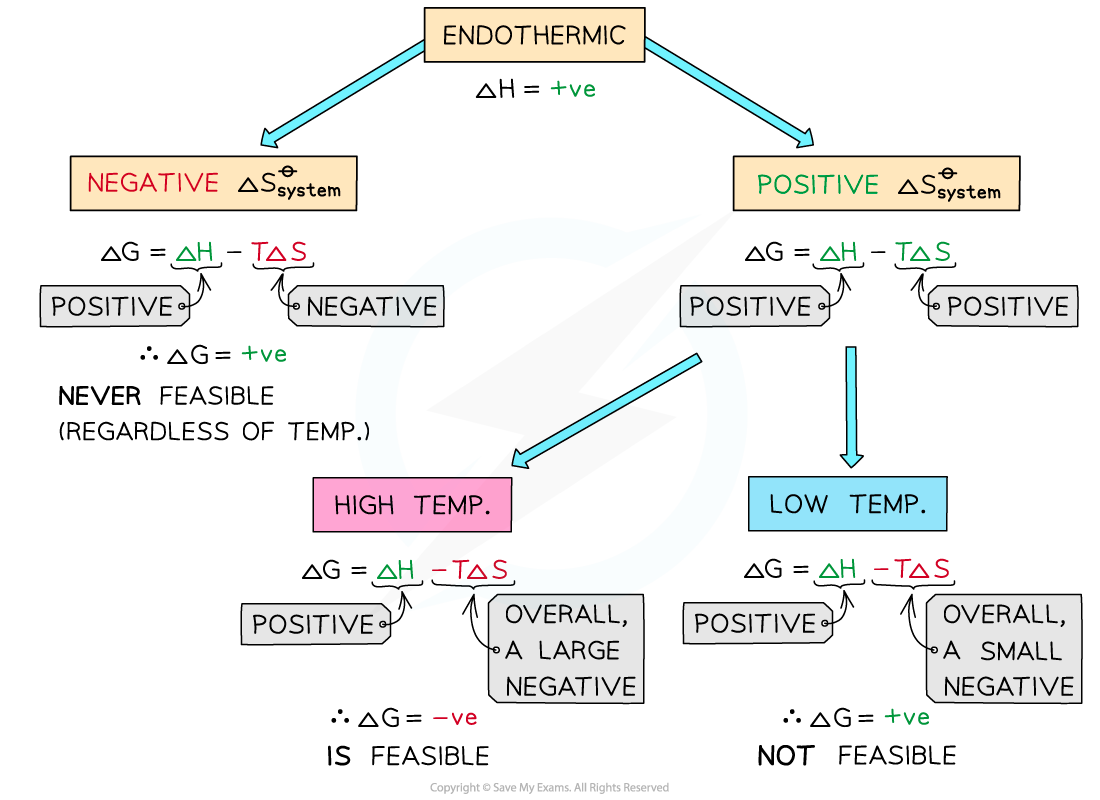
The diagram shows under which conditions endothermic reactions are feasible
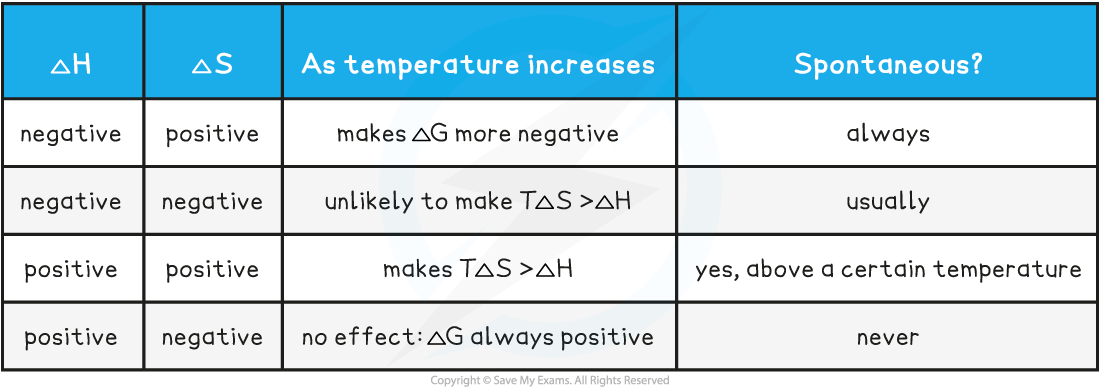
A summary table of free energy conditions
Free Energy Vs Temperature Graphs
- Rearranging the Gibbs equation allows you to determine the temperature at which a non-spontaneous reaction become feasible
ΔG??= ΔHreaction??- TΔSsystem?
- For a reaction to be feasible ΔG??must be zero or negative
0 = ΔH??- TΔS?
ΔH??= TΔS?
T= ΔH??/ ΔS?
Worked Example
At what temperature will the reduction of aluminium oxide with carbon become spontaneous?
Al2O3(s) + 3C(s)?? → ? 2Al(s) + 3CO(g)??? ? ? ? ? ?ΔH??= +1336?kJ mol-1? ? ? ??ΔS??= +581 J K-1mol-1
Answer:
If ΔG = 0 then ,? ?T= ΔH??/ ΔS?
T= 1336 ÷ (581/1000)
T= 2299 K
Graphing the Gibbs Equation
- The Gibbs equation can be expressed as the equation for a straight line
ΔG??= ΔH??- TΔS?
ΔG??=?-?ΔS?T+ ΔH?
y = mx + c
- A graph of free energy versus temperature (in K) will give a straight line, with slope -ΔS??and y-intercept, ΔH?.
- The variation of ΔG??against T for the synthesis of ammonia has been plotted below:
N2?(g) + 3H2?(g)? 2NH3?(g)
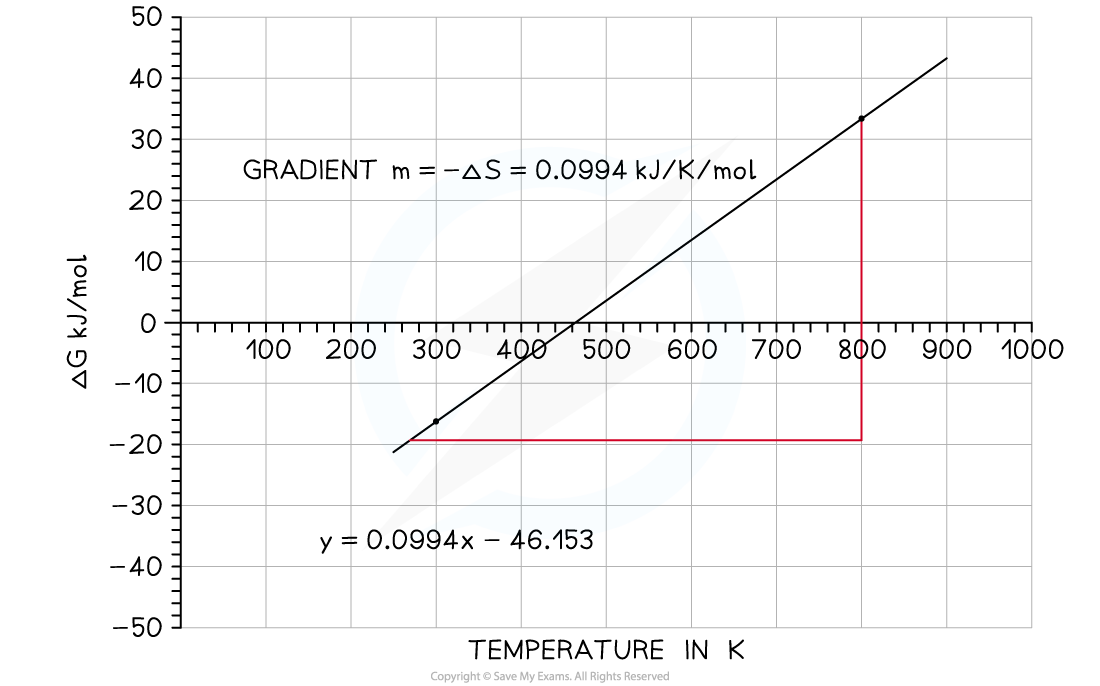
Graph of free energy versus temperature for the synthesis of ammonia
- From this graph you should be able to see some key features:
- The x-intercept shows you where the reaction ceases to be spontaneous, in this case at 460 K (187?oC)
- Above this temperature ΔG??is positive so the reaction is not feasible
- However, you may recall that the operating conditions of the Haber process are higher than this temperature, but this graph takes no account of the use of a catalyst which affects the energetics of the system, nor does it take into account anything about the rate of reaction or the fact that it is an equilibrium and removal of the ammonia as soon as it is formed also tips the balance in favour of the product
- The y-intercept shows you reaction is exothermic which you can see from the enthalpy of formation; the value is approximately -46 kJ mol?-1
- An exothermic equilibrium reaction would be favoured by lower temperatures - this is seen by the value of ΔG???becoming increasingly negative as the temperature falls
Exam Tip
You will notice that the line on the graph does not continue below 240 K. The simple reason for this is that at this point the ammonia will have reached it boiling point and so the gradient would change because it is now liquid ammonia.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









