- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
AQA A Level Chemistry復習筆記5.2.1 Rate Equations
Rate Equations
- The?rate of reaction?refers to the change in the amount or concentration of a reactant OR product per unit time
- It can be found by:
- Measuring the?decrease in the concentration of a reactant?over time
- Measuring the?increase in the concentration of a product?over time
- The units for rate of reaction are mol dm-3?s-1
Rate equation
- The following general reaction?will be used as an example to study the rate of reaction
D (aq) → E (aq) + F (g)
- The rate of reaction at different concentrations of D?is measured and tabulated
Rate of reactions table
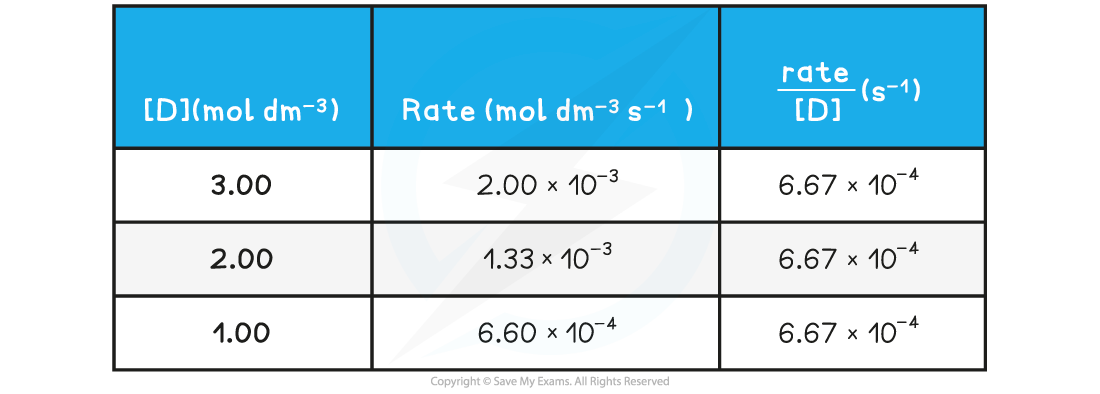
- A?directly proportional?relationship between the?rate?of the reaction and?concentration of D?is observed when a graph is plotted
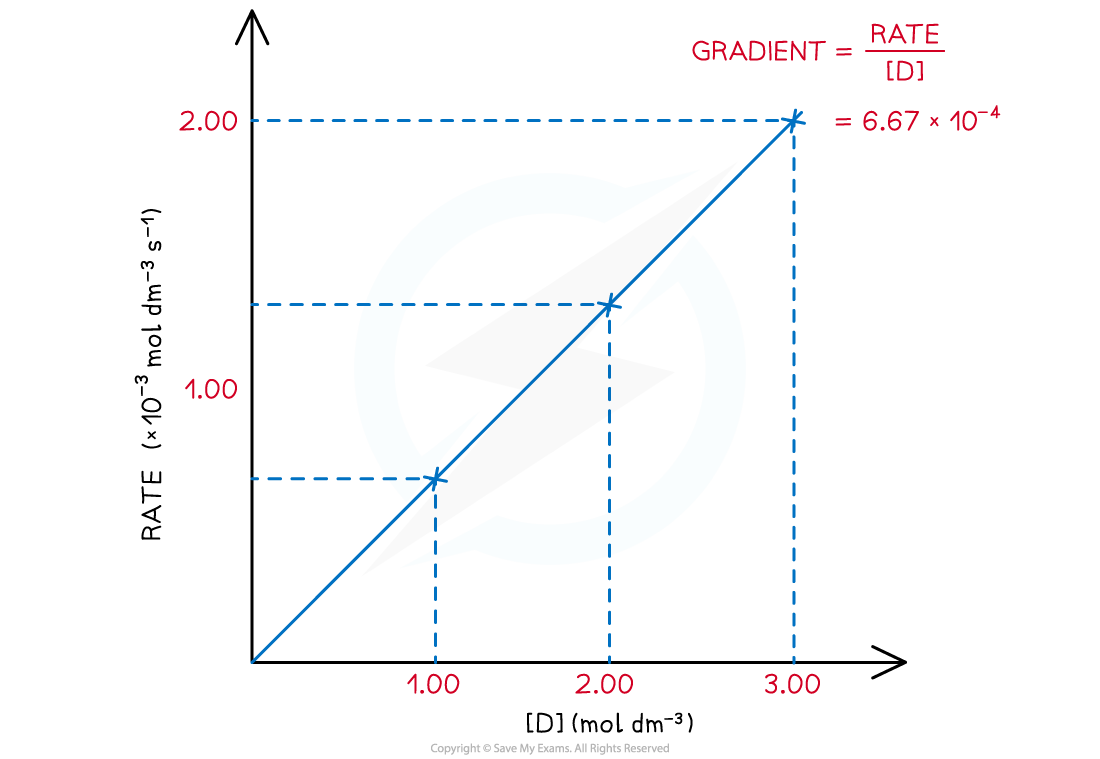
Rate of reaction over various concentrations of D
- Rate equations can?only?be determined?experimentally?and cannot be found from the?stoichiometric equations
Rate of reaction =?k?[A]m?[B]n
[A] and [B] = concentrations of reactants
m?and?n?= orders of the reaction
- All of the reactant concentrations will have an order of 0, 1 or 2, depending on the effect that they have on the rate of the reaction
- The products are never involved in the rate equation, as they have no effect on the rate of the reaction
- For the above reaction, the rate equation would beRate =?k?[D]
- Let's take a real life example:
2NO (g) + 2H2?(g) → N2?(g) + 2H2O (g)
- The?rate equation?for the formation of nitrogen gas (N2) from nitrogen oxide (NO) and hydrogen (H2) is:
rate =?k?[NO]2?[H2]
- Notice that the?[H2] does not have an order of 2
- This is because the order must be determined experimentally, not from the equation
- The orders of the reaction will be calculated from a table of experimental data, or from a graph
- The rate equation for the reaction above shows that:
- When changing the concentration of NO to determine how it affects the rate, while keeping [H2] constant
- The change in rate is?proportional to?the?square?of [NO]
Rate =?k1?[NO]2
- And, when changing the [H2] to determine how it affects the rate while keeping [NO] constant
- The change in rate is?proportional?to [H2]
Rate =?k2?[H2]
- Combining the two equations gives the?overall rate equation?(where?k?=?k1?+ k2)
Rate =?k?[NO]2?[H2]
- For a catalyst to appear in the rate equation:
- It must have a measurable and quantifiable effect on the rate of reaction
- The catalyst must be homogeneous
- If a chemical appears in a rate equation but is not one of the reactants, then it is a catalyst
Order of reaction
- The?order?of a reactant shows how the concentration of a reactant affects the rate of reaction
- It is the power to which the concentration of that reactant is raised in the rate equation
- The order can be 0, 1 or 2
- When the order of reaction of a reactant is 0, this means that it has no effect on the rate of the reaction and therefore is not included in the rate equation at all
- When the order of reaction of a reactant is 1, the rate is directly proportional to the concentration of that reactant
- When the order of reaction of a reactant is 2, the rate is directly proportional to the square of the concentration of that reactant
- The?overall order of reaction?is the sum of the powers of the reactants in a rate equation
- For example, in the following rate equation, the reaction is:
Rate =?k?[NO]2?[H2]
-
- Second-order with respect to NO
- First-order with respect to H2
- Third-order overall (2 + 1)
Half-life
- The?half-life (t1/2)?is the time taken for the concentration of a?limiting reactant?to become half of its initial value
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









