- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
AP化學真題
AP化學真題解析
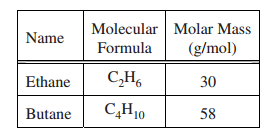
The molecular formula and molar mass of two straight-chain hydrocarbons arelisted in the table above. Based on the information in the table, which compoundhas the higher boiling point, and why is that compounds boiling pointhigher?
(A) C4H10, because it has more hydrogen atoms resulting in more hydrogen bonding
(B) C4H10, because it has more electrons, resulting in greater polarizability and stronger dispersion forces
(C) C2H6, because its molecules are smaller and they can get closer to one another, resulting in stronger dispersion forces(D) C2H6, because its molecules are more polar resulting in stronger dipole-dipole attractions
(題目選自2015 AP Chemistry Practice Exam Q11)
解析如下:
本道題目主要考察如何比較有機小分子沸點的高低。
物質的熔沸點,蒸氣壓等物理性質都是由分子間作用力( IMF, Intermolecular Forces)決定的: IMF越強,熔沸點就越高,所以題目旨在比較兩個有機小分子的IMF。
由于二者都是非極性(Non-polar)分子,所以二者分子間作用力都僅有dispersion force,而dispersion force取決于分子的極化率(polarizability),電子數目越多越容易極化,所以dispersionforce的強弱與非極性分子的大小成正比,所以答案為B。

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









