- 翰林提供學(xué)術(shù)活動、國際課程、科研項目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
2013 AP Chemistry化學(xué)真題系列之選擇題免費下載
歷年AP Calculus BC微積分BC系列
真題與答案下載

翰林國際教育全網(wǎng)首發(fā)
力爭超快速發(fā)布最全資料
助你在升學(xué)路上一帆風(fēng)順
為你的未來保駕護航
2013 AP Chemistry Practice Exam Multiple Choice Free Download
2013 AP 化學(xué)模考選擇題部分免費下載
此套Section I試卷共分計時1小時30分鐘組成
共60題
僅限使用鉛筆,無計算器
考試時會提供花常用的等式與常量
以及化學(xué)元素周期表
完整版下載鏈接見文末
部分真題預(yù)覽:
1)Complete combustion of a sample of a hydrocarbon in excess oxygen produces equimolar quantities of carbon dioxide and water. Which of the following could be the molecular formula of the compound?
C2H2
C2H6
C4H8
C6H6
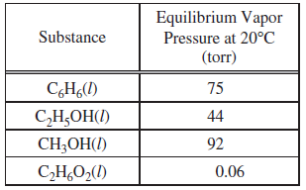
2)Based on the data in the table above, which of the following liquid substances has the weakest intermolecular forces?
C6H6(l)
C2H5OH(l)
CH3OH(l)
C2H6O2(l)

3)Based on the data in the table above, which of the following correctly predicts the relative strength of the attraction of Zn2+, Ca2+, and Ba2+ ions to water molecules in a solution, from strongest to weakest, and provides the correct reason?
Zn2+ > Ca2+ > Ba2+ because the smaller ions have a stronger coulombic attraction to water
Zn2+ > Ca2+ > Ba2+ because the smaller ions are more electronegative
Ba2+ > Ca2+ > Zn2+ because the larger ions are more polarizable
Ba2+ > Ca2+ > Zn2+ because the larger ions are less electronegative
4)Zn(s) is used to reduce other compounds in chemical reactions. If a chemist needs a substance that is more effective in its reducing ability, which of the following species would be the best choice?
Na
H+
K+
Cl?
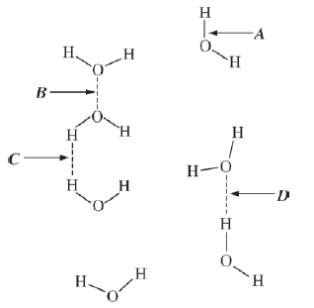
5) In the diagram above, which of the labeled arrows identifies hydrogen bonding in water?
A
B
C
D
6) A kinetics experiment is set up to collect the gas that is generated when a sample of chalk, consisting primarily of solid CaCO3 , is added to a solution of ethanoic acid, CH3COOH. The rate of reaction between CaCO3 and CH3COOH is determined by measuring the volume of gas generated at 25°C and 1 atm as a function of time. Which of the following experimental conditions is most likely to increase the rate of gas production?
Decreasing the volume of ethanoic acid solution used in the experiment
Decreasing the concentration of the ethanoic acid solution used in the experiment
Decreasing the temperature at which the experiment is performed
Decreasing the particle size of the CaCO3 by grinding it into a fine powder
2013 AP Chemistry化學(xué)模考MC選擇題完整版答案免費下載
請持續(xù)關(guān)注,稍后更新
2013 AP Chemistry Sample Exam Multiple Choice Free Download
2013 AP 化學(xué)樣卷選擇題部分免費下載
此套Section I試卷共分計時1小時30分鐘組成
共75題
僅限使用鉛筆,無計算器
考試時會提供花常用的等式與常量
以及化學(xué)元素周期表
完整版真題資料可以底部二維碼免費領(lǐng)取↓↓↓

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









