- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
2008 AP Chemistry化學真題系列之簡答題免費下載
歷年AP Chemistry化學系列
真題與答案下載

翰林國際教育全網首發
力爭超快速發布最全資料
助你在升學路上一帆風順
為你的未來保駕護航
2008 AP Chemistry Free-Response Questions Free Download
2008 AP 化學簡答題部分免費下載
考試時會提供花常用的等式與常量
以及化學元素周期表
此套Section II試卷共6題
每道大題含有不同數量的小題
共計時95分鐘
Part A計55分鐘,第1-3題各20分
Part B計40分鐘,第四題值10分,第5第6題各15分
完整版下載鏈接見文末
部分真題預覽:
Section II, Part A,YOU MAY USE YOUR CALCULATOR FOR PART A可使用計算器:
![]()
1)Solid carbon and carbon dioxide gas at 1,160 K were placed in a rigid 2.00 L container, and the reaction represented above occurred. As the reaction proceeded, the total pressure in the container was monitored. When equilibrium was reached, there was still some C(s) remaining in the container. Results are recorded
in the table below.
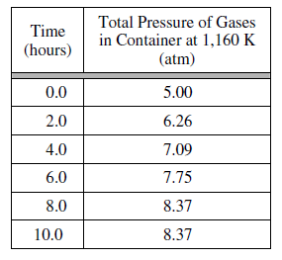
- Write the expression for the equilibrium constant, Kp , for the reaction.
- Calculate the number of moles of CO2(g) initially placed in the container. (Assume that the volume of the solid carbon is negligible.)
- For the reaction mixture at equilibrium at 1,160 K, the partial pressure of the CO2(g) is 1.63 atm. Calculate
- the partial pressure of CO(g) , and
- the value of the equilibrium constant, Kp .
- If a suitable solid catalyst were placed in the reaction vessel, would the final total pressure of the gases at equilibrium be greater than, less than, or equal to the final total pressure of the gases at equilibrium without the catalyst? Justify your answer. (Assume that the volume of the solid catalyst is negligible.)
In another experiment involving the same reaction, a rigid 2.00 L container initially contains 10.0 g of C(s) , plus CO(g) and CO2(g) , each at a partial pressure of 2.00 atm at 1,160 K.
f. Predict whether the partial pressure of CO2(g) will increase, decrease, or remain the same as this system approaches equilibrium. Justify your prediction with a calculation.
完整版真題下載鏈接請注冊或登錄后查看
文件為PDF格式
推薦使用電腦下載
2008 AP Chemistry化學 FRQ簡答題完整版答案免費下載
請持續關注,稍后更新
2008 AP Chemistry Free-Response Questions(Form B) Free Download
2008 AP 化學簡答題(Form B)部分免費下載
考試時會提供花常用的等式與常量
以及化學元素周期表
此套Section II試卷共6題
共計時95分鐘
下載方式請點擊下方鏈接
2008 AP Chemistry (Form B)FRQ真題題目
翰林學員全站資料免費打包下載,專享高速下載通道。
[vc_btn title="查看更多AP Chemistry化學課程相關詳情" color="primary" align="center" i_icon_fontawesome="fa fa-globe" css_animation="zoomIn" button_block="true" add_icon="true" link="url:http%3A%2F%2Fwww.linstitute.net%2Farchives%2F25860||target:%20_blank|"]

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









