- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Edexcel IGCSE Physics: Double Science 復習筆記:5.2.3 Temperature
Edexcel IGCSE Physics: Double Science 復習筆記:5.2.3 Temperature
Temperature & Speed
- Imagine molecules of gas that are free to move around in a box
- The molecules in the gas move around randomly at high speeds, colliding with surfaces and exerting pressure upon them
- The?temperature?of a gas is related to the?average speed?of the molecules:
- The?hotter?the gas, the?faster?the molecules move and vice versa
- Hence, the molecules collide with the surface of the walls?more frequently

Gas molecules move about randomly at high speeds
- This is because their?kinetic energy?increases
Temperature & Kinetic Energy
- Heating a system will change the energy stored in a system by?increasing the kinetic energy?of its particles
- The Kelvin?temperature?of the gas is related to the average?kinetic energy?of the molecules
- This increase in kinetic energy (and therefore energy stored in the system) can:
- Cause the?temperature?of the system to increase
- Or, produce a?change of state?(solid to liquid or liquid to gas)
- The internal energy of a gas is the sum of the kinetic energy of all the molecules
- The higher the temperature, the higher the average kinetic energy of the molecules and vice versa
- This means they move around?faster
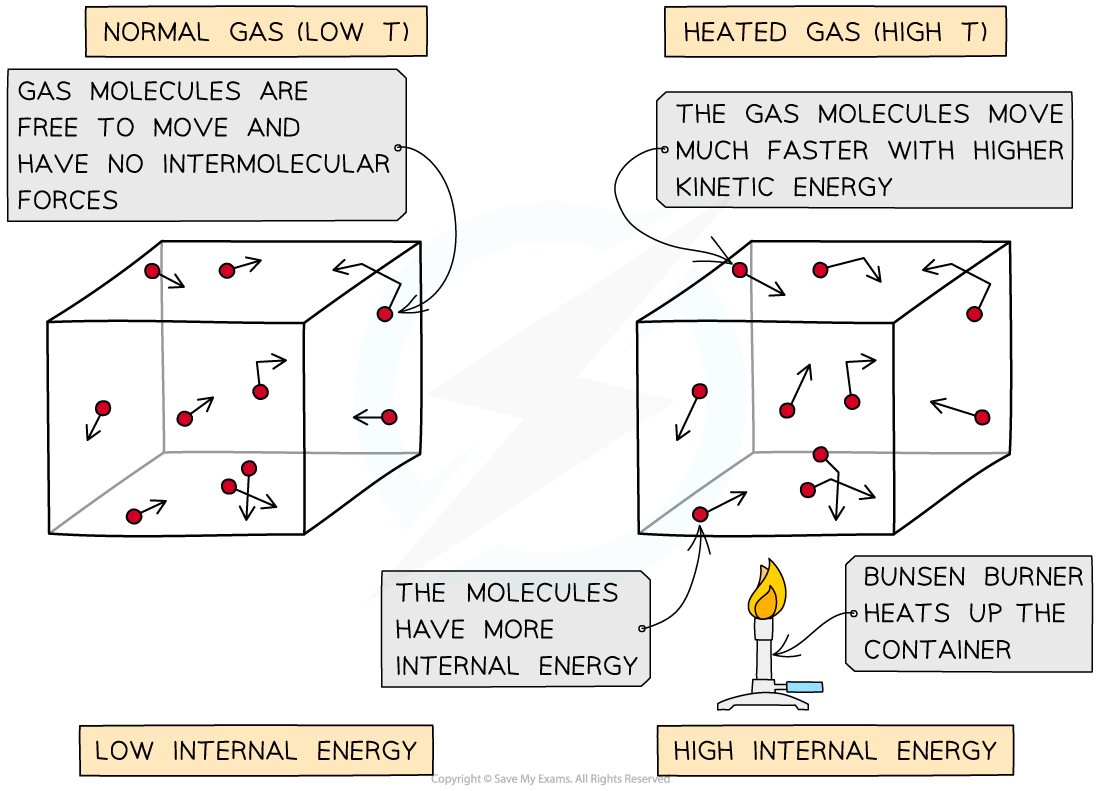
As the container is heated up, the gas molecules move faster with higher kinetic energy. The energy stored within the system - the internal energy - therefore increases
- If the temperature of a gas is increased, the particles move faster and gain?kinetic energy
- Therefore, they will collide more with each other and the container leading to an increase in pressure
- The temperature (in Kelvin) is?proportional?to the average kinetic energy of the molecules
T ∝ KE
Worked Example
When a liquid evaporates, molecules escape from the surface of the liquid. What happens to the temperature of the liquid and the average kinetic energy of the molecules within it?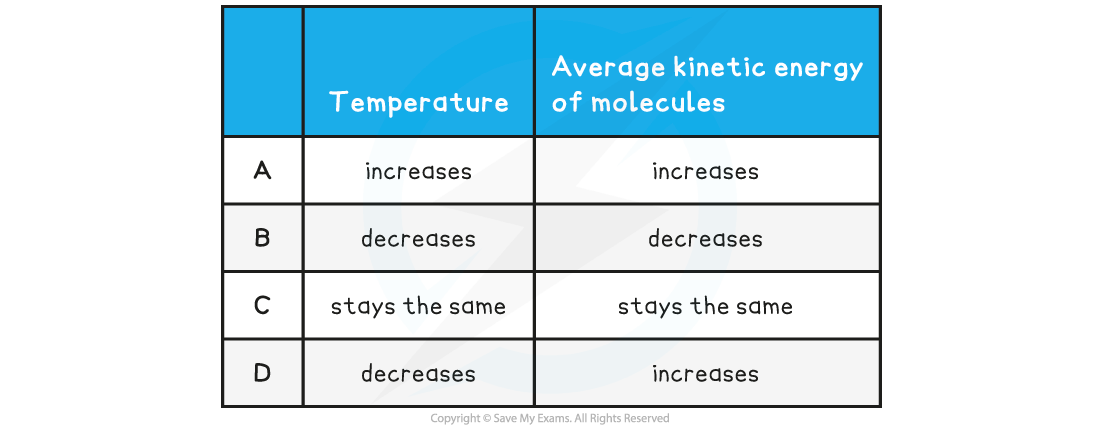
ANSWER:? B
-
- When evaporation takes place, the more energetic molecules are leaving the surface of the liquid
- Since the more energetic molecules have left, the?average kinetic energy per molecule?must?decrease
- Therefore,?A, C?&?D?are not correct
- Temperature is?proportional?to the average kinetic energy per molecule, therefore the?temperature?also?decreases
轉載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









