- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Edexcel A Level Physics:復習筆記9.9 Average Molecular Kinetic Energy
Average Molecular Kinetic Energy
- An important property of molecules in a gas is their?average kinetic energy
- This can be deduced from the ideal gas equations relating pressure, volume, temperature and speed
- Recall the ideal gas equation in terms of number of molecules:
pV?=?NkT
- Also, recall the equation linking pressure and mean square speed of the molecules:
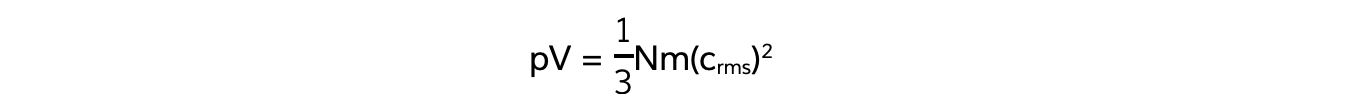
- The left-hand side of both equations are equal to pV
- This means the right-hand sides of both equations are also equal:
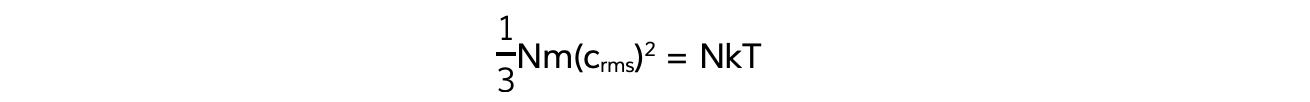
- N will cancel out on both sides and multiplying by 3 on both sides too obtains the equation:
m(crms)2?= 3kT
- Recall the familiar kinetic energy equation from mechanics:

- Instead of v2?for the velocity of one particle, (crms)2?is the average speed of all molecules
- Multiplying both sides of the equation by ? obtains the?average molecular kinetic energy?of the molecules of an ideal gas:
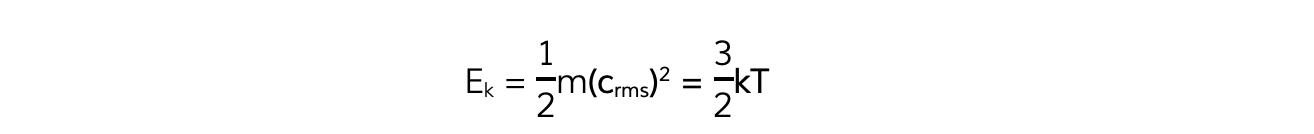
- Where:
- Ek?= kinetic energy of a molecule (J)
- m?= mass of one molecule (kg)
- ?(crms)2?= mean square speed of a molecule (m2?s-2)
- k?=?Boltzmann constant
- T?= temperature of the gas (K)
- Note:?this is the average kinetic energy for only?one?molecule of the gas
- To find the average kinetic energy for many molecules of the gas, multiply both sides of the equation by the number of molecules N to obtain:
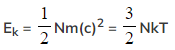
- A key feature of this equation is that the mean kinetic energy of an ideal gas molecule is proportional to its thermodynamic temperature
Ek?∝?T
- The Boltzmann constant?k?can be replaced with
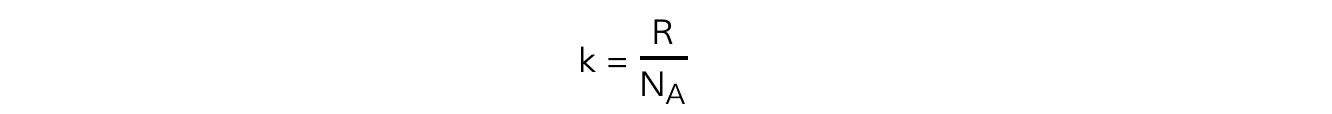
- Substituting this into the average molecular kinetic energy equation means it can also be written as:
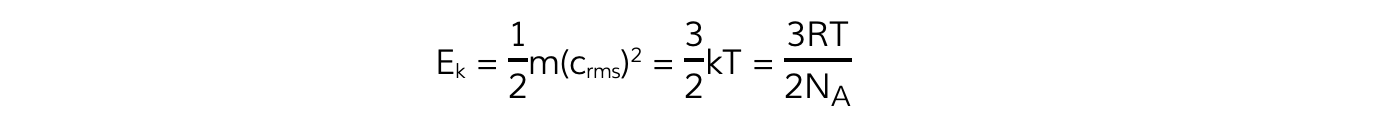
Worked Example
Helium can be treated as an ideal gas. Helium molecules have a root-mean-square (r.m.s.) speed of 730 m s-1?at a temperature of 45 °C. Calculate the r.m.s. speed of the molecules at a temperature of 80 °C.
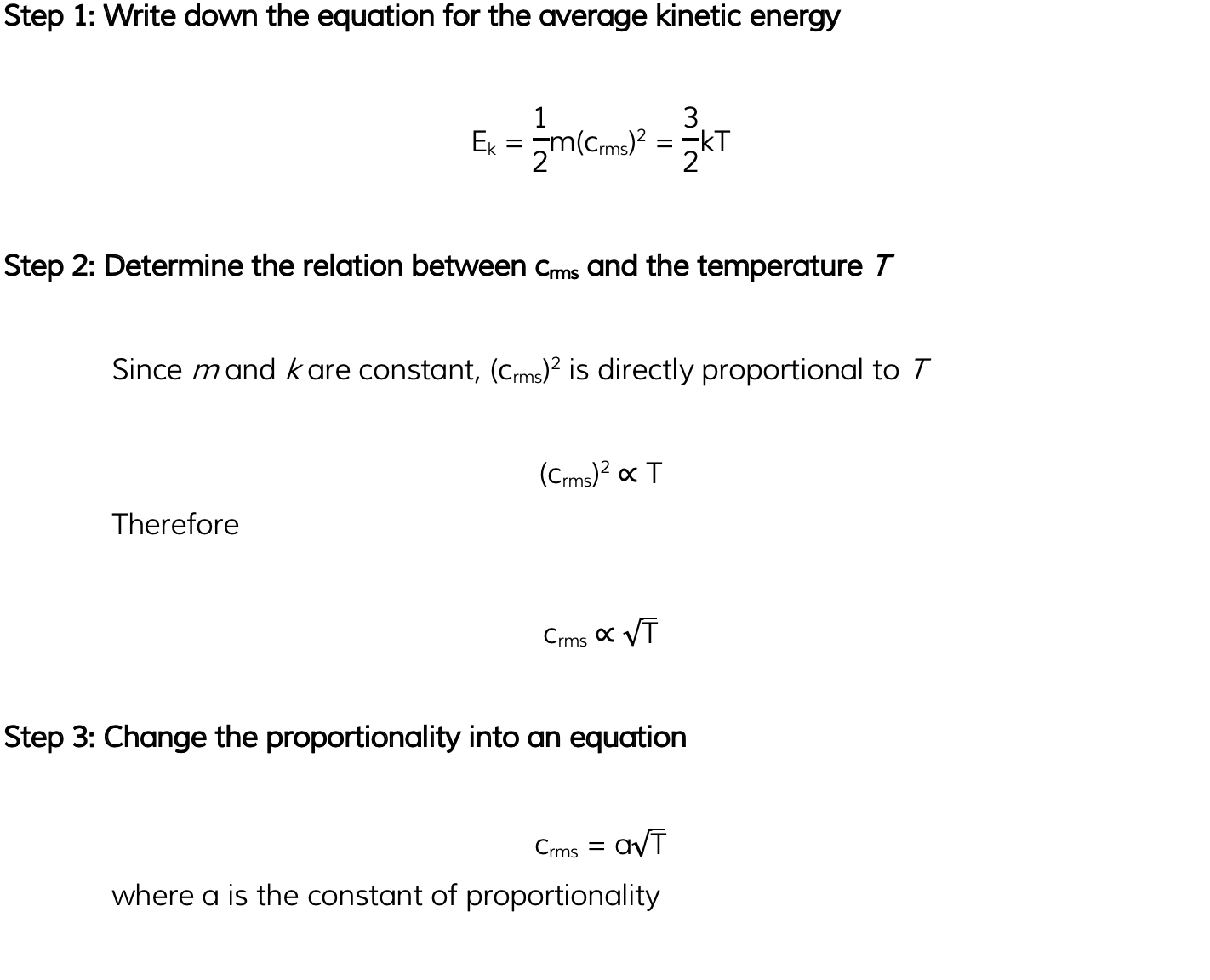
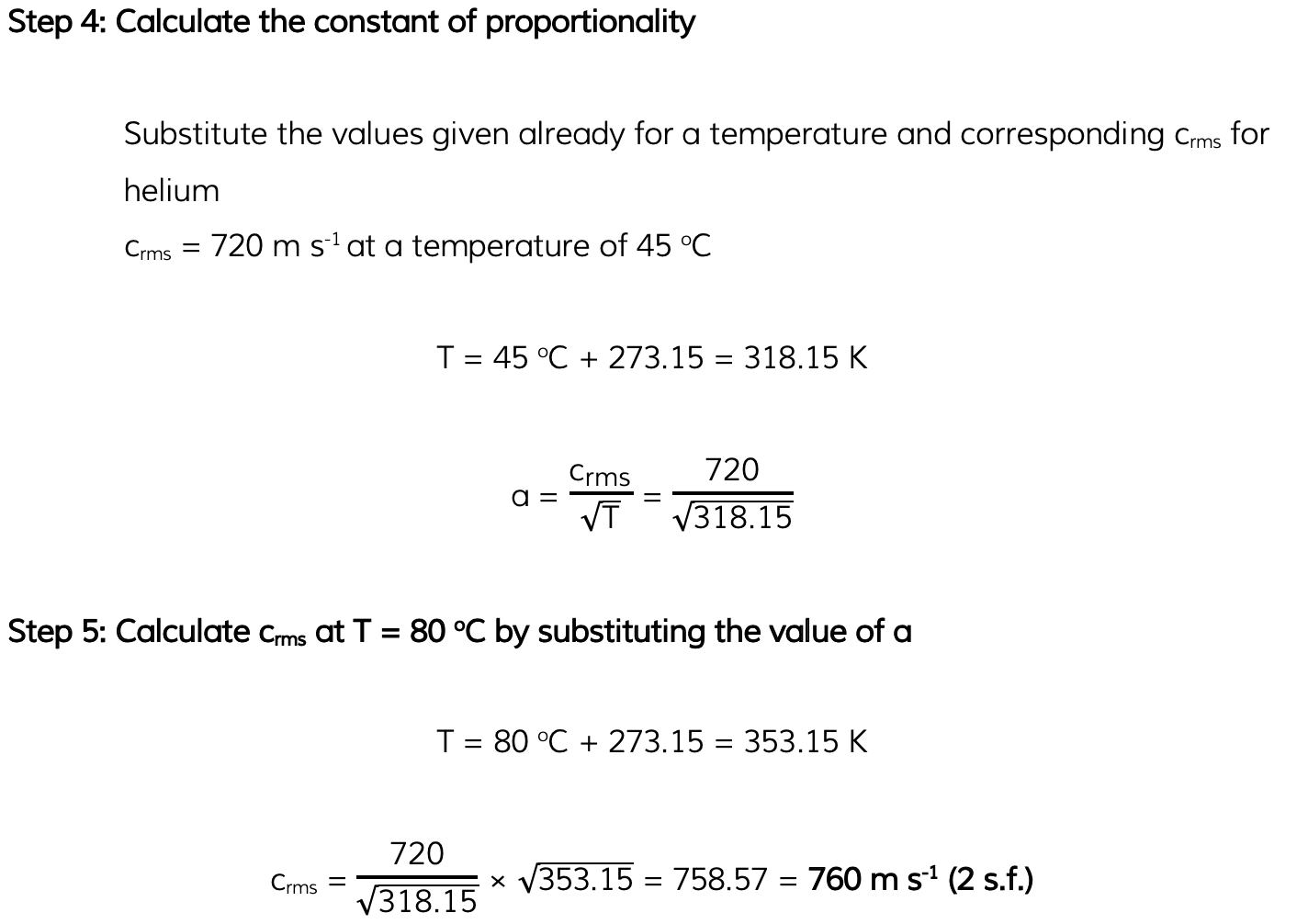
Exam Tip
You can remember the equation through the rhyme ‘Average K.E is three-halves kT’.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









