- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Edexcel A Level Chemistry:復習筆記7.4.1 Benzene Structure
Models of Benzene
Structure of Benzene
- The structure of benzene was determined many years ago, by a chemist called Kekule
- The structure consists of 6 carbon atoms in a hexagonal ring, with alternating single and double carbon-carbon bonds
- This suggests that benzene should react in the same way that an unsaturated alkene does
- However, this is not the case
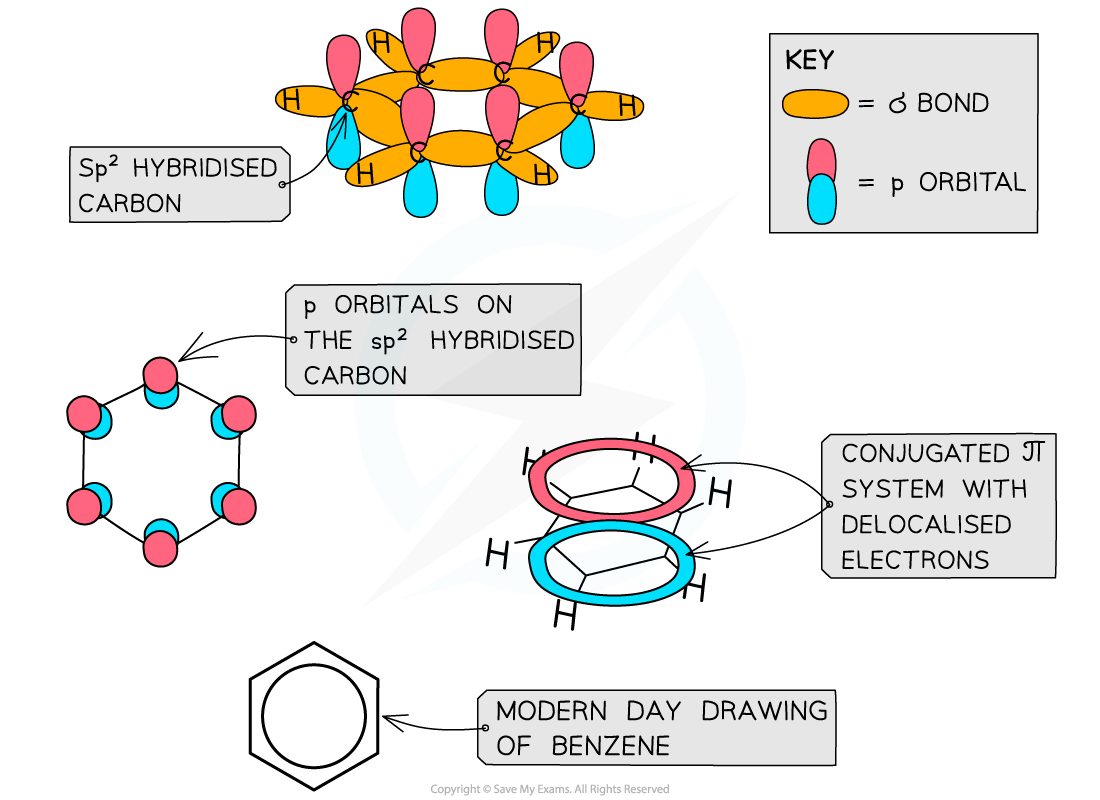
Like other aromatic compounds, benzene has a planar structure due to the sp2?hybridisation of carbon atoms and the conjugated π system in the ring
- Each carbon atom in the ring forms three σ bonds using the sp2?orbitals
- The remaining?p orbitals?overlap laterally with p orbitals of neighbouring carbon atoms to form a π system
- This extensive sideways overlap of p orbitals results in the electrons being delocalised and able to freely spread over the entire ring causing a π system
- The π system is made up of two ring shaped clouds of electron density - one above the plane and one below it
- Benzene and other aromatic compounds are?regular?and?planar?compounds with bond angles of 120?o
- The delocalisation of electrons means that all of the carbon-carbon bonds in these compounds are identical and have both?single?and?double bond?character
- The bonds all being the same length is evidence for the delocalised ring structure of benzene
Evidence for delocalisation
- This evidence of the bonding in benzene is provided by data from enthalpy changes of hydrogenation and carbon-carbon bond lengths
- Hydrogenation of cyclohexene
- Each molecule has one C=C double bond
- The enthalpy change for the reaction of cyclohexene is -120 kJ mol-1
C6H10?+ H2?→?C6H12???ΔHΘ?= -120?kJ mol-1
- Hydrogenation of beznene
- The Kekule structure of benzene as cyclohexa-1,3,5-triene has three double C=C bonds
- It would be expected that the enthalpy change for the hydrogenation of this structure would be three times the enthalpy change for the one C=C bond in cyclohexene
C6H6?+ 3H2?→?C6H12???ΔHΘ?= 3 x -120?kJ mol-1?= -360?kJ mol-1
- When benzene is reacted with hydrogen, the enthalpy change obtained is actually far less exothermic,?ΔHΘ?= -208 kJ mol-1
Resistance to Bromination
- Alkenes tend to undergo bromination easily which can be observed in cyclohexene
C6H10?+ Br2?→?C6H10Br2
- As the π bond contains localised electrons, it produces an area of high electron density allowing it to repel the electron in the bromine molecule
- Therefore a dipole is introduced making one bromine atom δ+ and one δ- bromine atom
- The δ+ bromine is attracted to the π bond in the cyclohexane
- This then leaves a carbocation in the intermediate molecule which the negative bromide ion is attracted to, hence forming 1,2-dibromocyclohexane by electrophilic addition
- In benzene, there are no localised areas of high electron density, preventing it from being able to polarise the bromine moelcule
- In order for benzene to undergo electrophilic substitution with bromine, a halogen carrier must be present in the reaction e.g. AlBr3
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









