- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Edexcel A Level Chemistry:復習筆記4.1.3 Determining Concentrations
Core Practical 3: Hydrochloric Acid Concentration
Performing the Titration
- The key piece of equipment used in the titration is the burette
- Burettes?are usually marked to a precision of 0.10 cm3
- Since they are analogue instruments, the uncertainty is recorded to?half?the smallest marking, in other words to ±0.05 cm3
- The?end point?or?equivalence point?occurs when the two solutions have reacted completely and is shown with the use of an?indicator
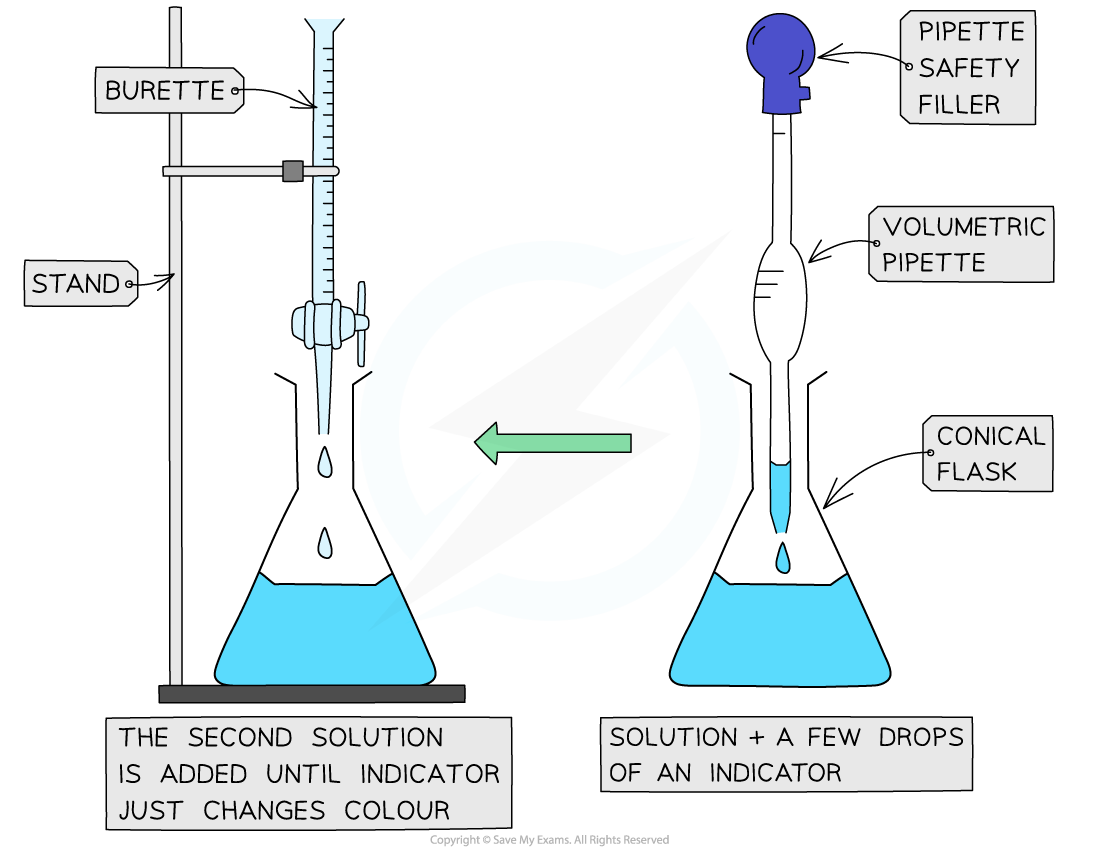
The steps in a titration
- A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour change
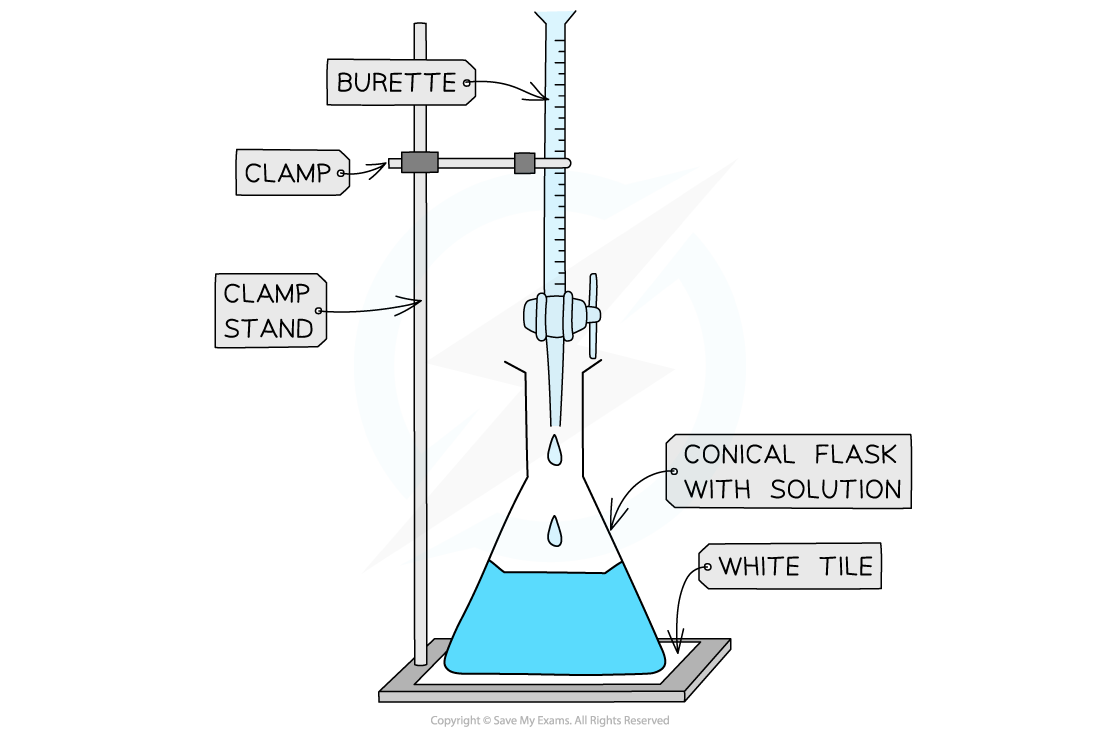
Titrating
- The steps in a titration are:
- Measuring a known volume (usually 20 or 25 cm3) of one of the solutions with a?volumetric?pipette?and placing it into a?conical flask
- The other solution is placed in the?burette
- To start with, the burette will usually be filled to 0.00 cm3
- A few drops of the?indicator?are added to the solution in the conical flask
- The tap on the?burette?is carefully opened and the solution added, portion by portion, to the?conical flask?until the?indicator?starts to change colour
- As you start getting near to the end point, the flow of the burette should be slowed right down so that the solution is added dropwise
- You should be able to close the tap on the burette after one drop has caused the colour change
- Multiple runs are carried out until?concordant?results are obtained
- Concordant results are within 0.1?cm3?of each other
Recording and processing titration results
- Both the initial and final?burette?readings should be recorded and shown to a?precision?of ?±0.05 cm3, the same as the?uncertainty
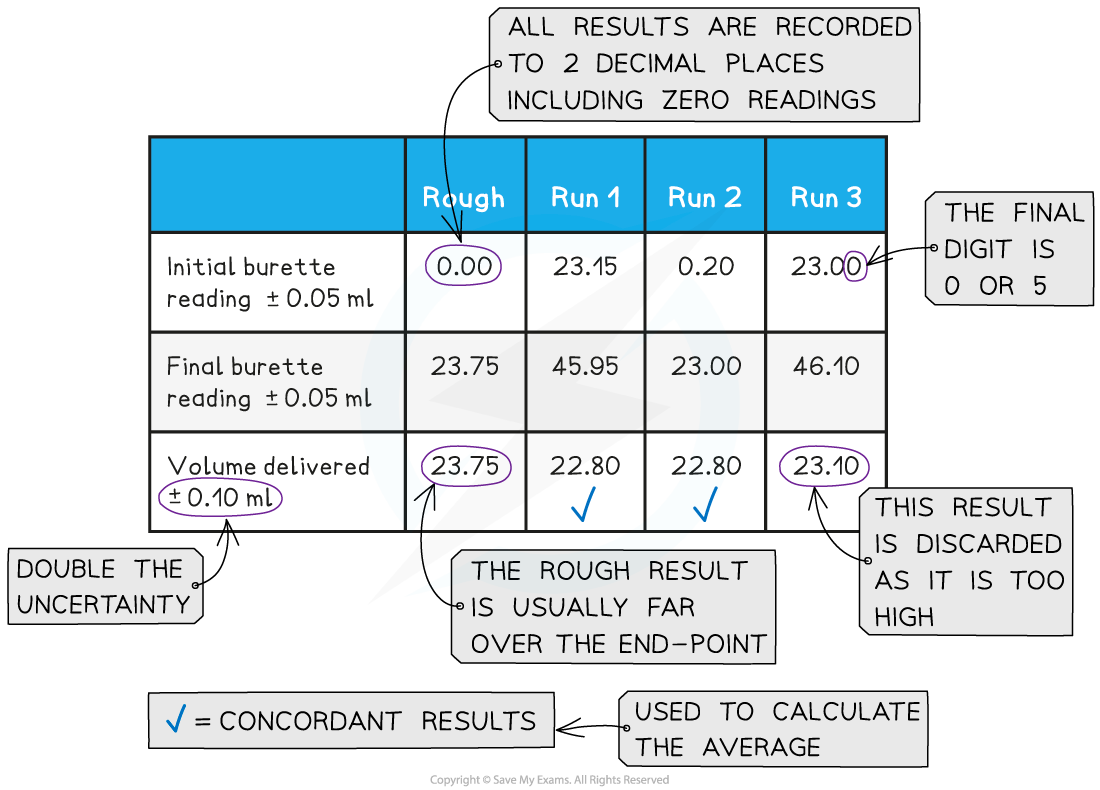
A typical layout and set of titration results
- The volume delivered (titre) is calculated and recorded to an?uncertainty?of ±0.10 cm3
- The?uncertainty?is doubled, because two?burette?readings are made to obtain the?titre?(V final – V initial), following the rules for?propagation of uncertainties
- Concordant?results are then averaged, and non-concordant results are discarded
- The appropriate calculations are then done
Worked Example
25.0 cm3?of?hydrochloric acid was titrated with a 0.200 mol dm-3?solution of sodium hydrogencarbonate, NaHCO3.
NaHCO3?+ HCl → NaCl + H2O + CO2
Use the following results to calculate the concentration of the acid, to 3 significant figures.
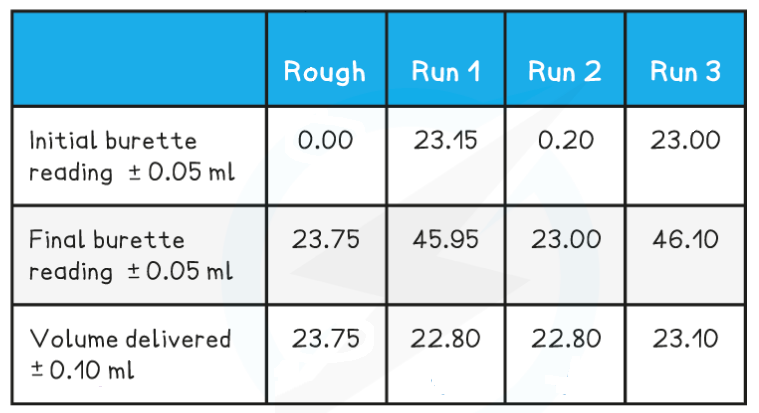
Answer
Step 1:?Calculate the average titre
-
- Average titre
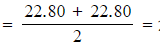 22.80 cm3
22.80 cm3
- Average titre
Step 2:?Calculate the number of moles of sodium hydrogencarbonate
-
- Moles
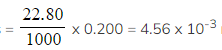 ?moles
?moles
- Moles
Step 3:?Calculate (or deduce) the number of moles of hydrochloric acid
-
- The stoichiometry of NaHCO3?: HCl is 1 : 1
- Therefore, the number of moles of sodium hydrogencarbonate is also 4.56 x 10-3?moles
Step 4:?Calculate the concentration of hydrochloric acid
-
- Concentration?
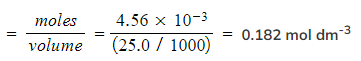
- Concentration?
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









