- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Edexcel A Level Chemistry:復習筆記3.3.4 Electrophilic Addition Mechanism
Electrophilic Addition Mechanism
Electrophilic addition of hydrogen halides
- Hydrogen halides such as hydrogen bromide (HBr) are polar as the hydrogen and halogen atoms have different electronegativities
- The bromine atom has a stronger pull on the electrons in the H-Br bond
- As a result of this, the Br atom has a partial negative and the H atom a partial positive charge
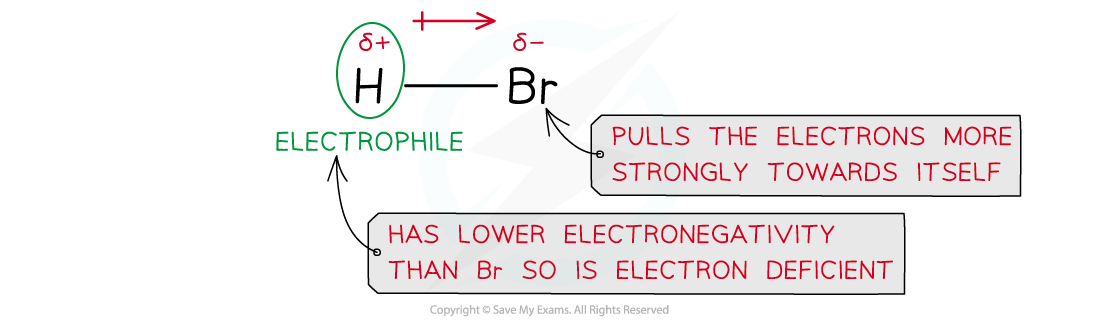
Due to differences in electronegativities of the hydrogen and bromine atom, HBr is a polar molecule
- In an addition reaction, the H atom acts as an electrophile and accepts a pair of electrons from the C-C bond in the alkene
- The H-Br bond breaks heterolytically, forming a Br-?ion
- This result in the formation of a highly reactive carbocation intermediate which reacts with the Br-?(nucleophile)
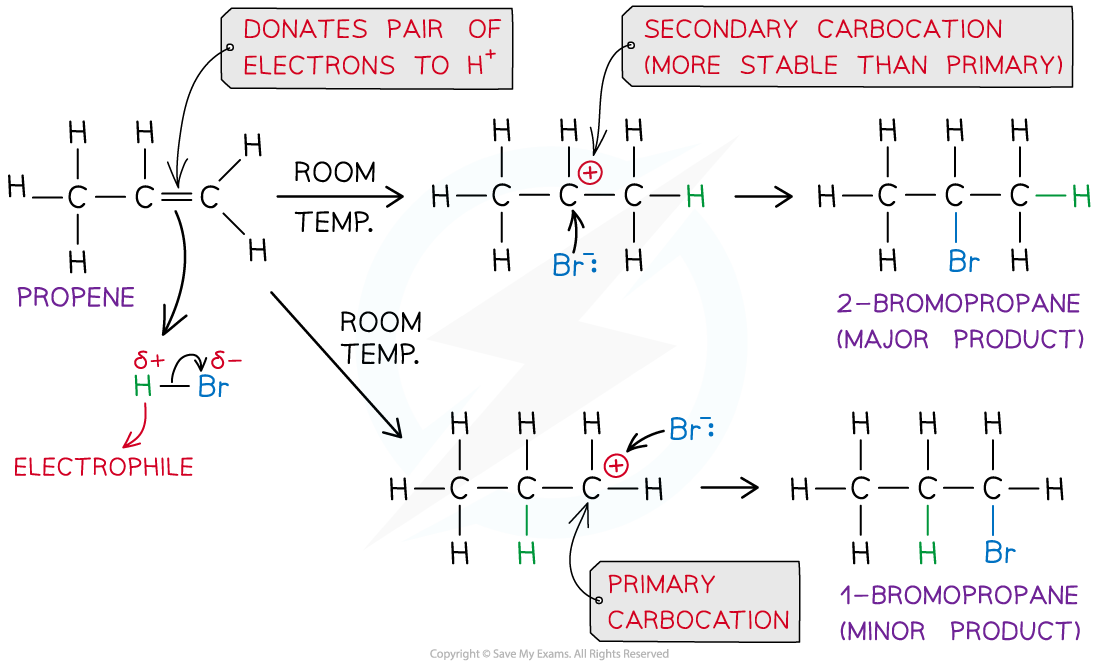
Example of an electrophilic addition reaction of HBr and propene to form 1-bromopropane and 2-bromopropane
Electrophilic addition of Halogens
- Halogens such as bromine (Br2) are a non-polar molecules as both atoms have similar electronegativities and therefore equally share the electrons in the covalent bond
- However, when a bromine molecule gets closer to the double bond of an alkene, the high electron density in the double bond repels the electron pair in Br-Br away from the closest Br atom
- As a result of this, the closest Br atom to the double bond is slightly positive and the further Br atom is slightly negatively charged
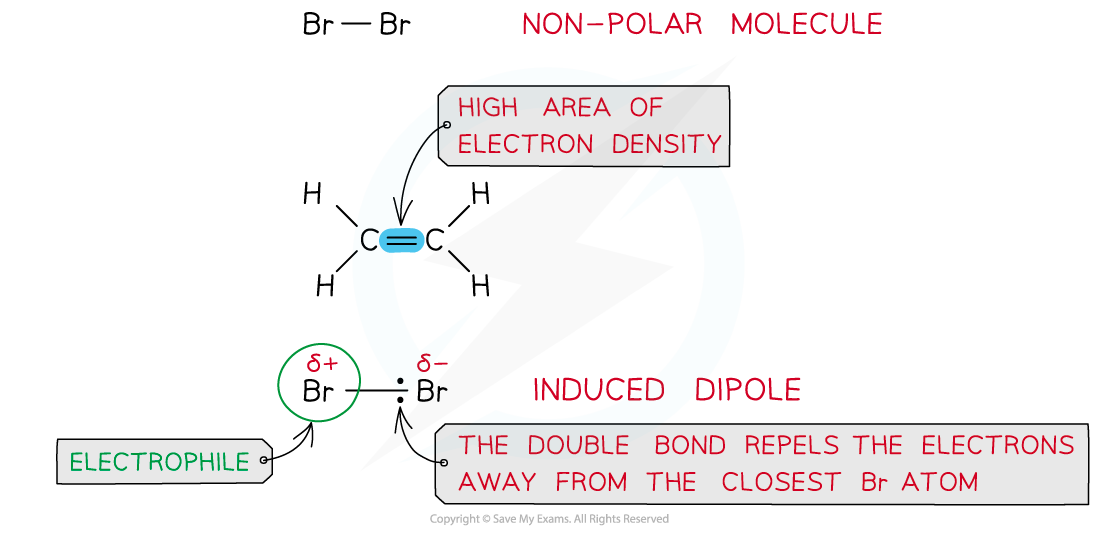
Br2?is a non-polar molecule however when placed close to an area of high electron density it can get polarised
- In an addition reaction, the closest Br atom acts as an electrophile and accepts a pair of electrons from the C-C bond in the alkene
- The Br-Br bond breaks heterolytically, forming a Br-?ion
- This results in the formation of a highly reactive carbocation intermediate which reacts with the :Br-?(nucleophile)
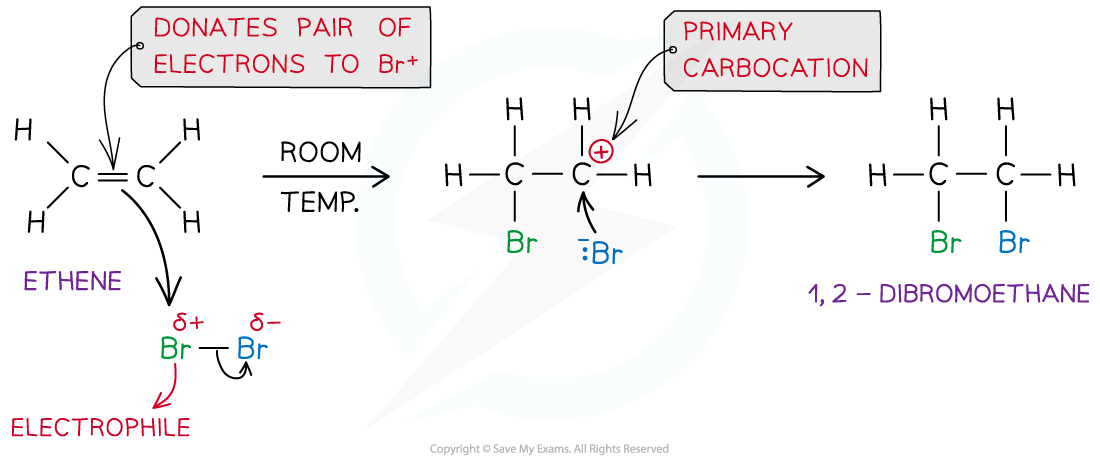
Example of an electrophilic addition reaction of Br2?and ethene to form dibromoethane
Exam Tip
The stability of the carbocation intermediate is as follows:
tertiary > secondary > primary
When more than one carbocations can be formed, the major product of the reaction will be the one that results from the nucleophilic attack of the most stable carbocation.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









