- 翰林提供學(xué)術(shù)活動(dòng)、國際課程、科研項(xiàng)目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
CIE A Level Chemistry復(fù)習(xí)筆記7.1.5 Shape of Aromatic Molecules
Shape of Benzene & Aromatic Molecules
- Aromatic molecules?consist of one or more rings with?conjugated?π systems
- Conjugated π systems?arise from alternating double and single bonds in which the electrons are?delocalised
- Aromatic compounds are called ‘a(chǎn)romatic’ as they often have pleasant odours
?Examples of aromatic compounds including benzene table
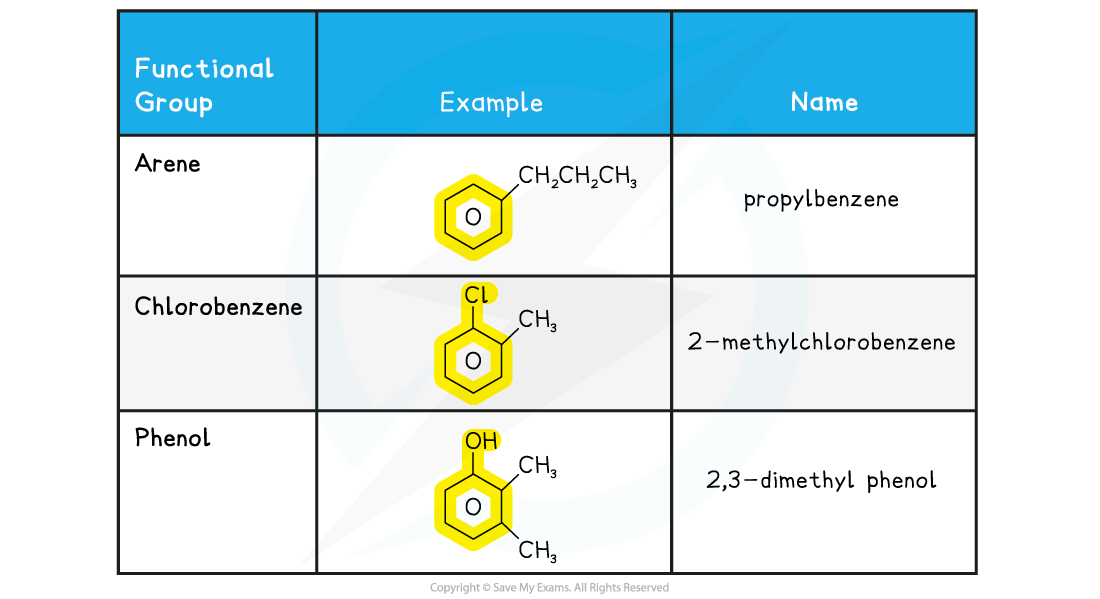
Shape of benzene & aromatic compounds
- Benzene and other aromatic compounds contain sp2?hybridised carbons as?two?of their?p?orbitals have mixed with an s orbital
- This means that each carbon atom in benzene and other aromatic compounds has?one p orbital
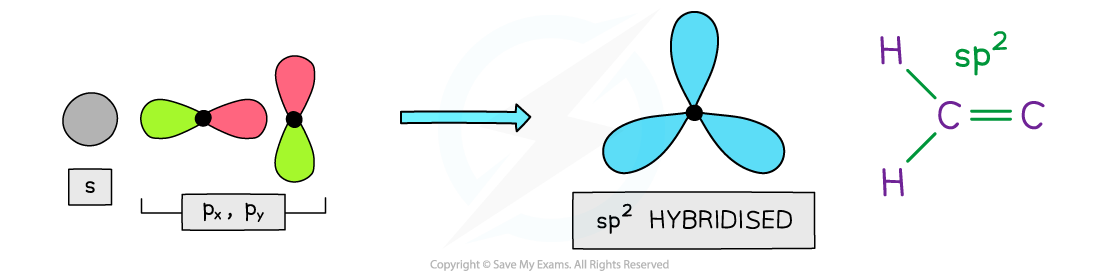
The carbon atoms in aromatic compounds are sp2?hybridized as two of their p orbitals mix with an s orbital
- Each carbon atom in the ring forms three σ bonds using the sp2?orbitals
- The remaining?p orbital?overlaps laterally with p orbitals of neighbouring carbon atoms to form a π bond
- This extensive sideways overlap of p orbitals results in the electrons being delocalised and able to freely spread over the entire ring
- Benzene and other aromatic compounds are?regular?and?planar?compounds with bond angles of 120o
- The delocalisation of electrons means that all of the carbon-carbon bonds in these compounds are identical and have both?single?and?double bond?character
- The bonds all being the same length is evidence for the delocalised ring structure of benzene
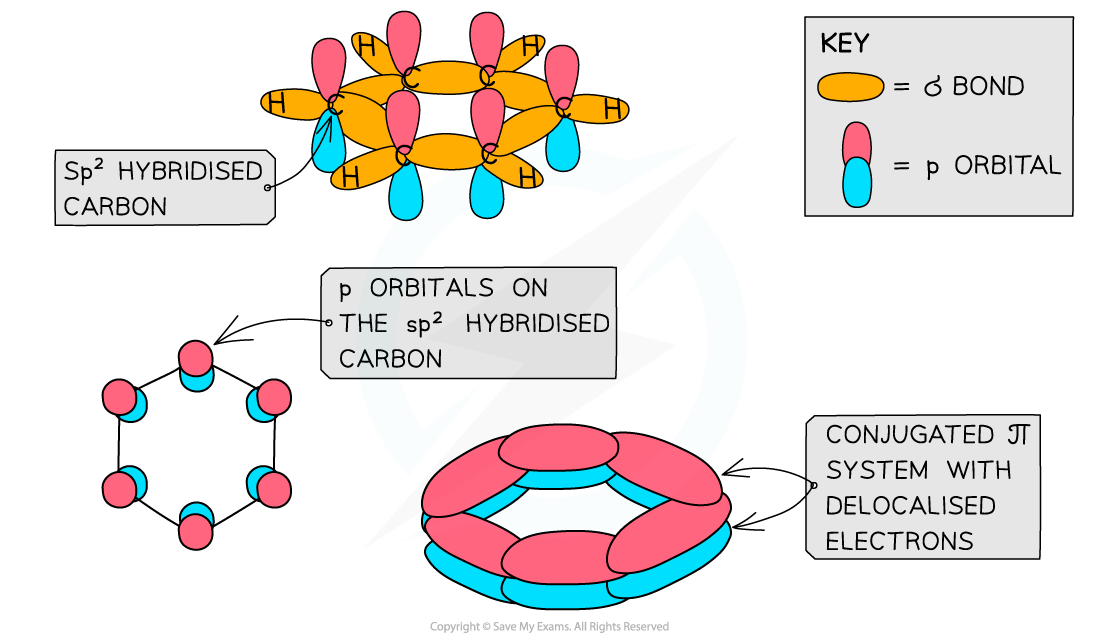
Like other aromatic compounds, benzene has a planar structure due to the sp2?hybridisation of carbon atoms and the conjugated π system in the ring
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號(hào)-1









