- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記6.1.1 Similarities, Trends & Compounds of Magnesium to Barium
Effect of Ionic Radius on Thermal Stability of Group 2 Nitrates & Carbonates
- The Group 2 nitrates and carbonates become?more thermally stable?going down the group
- The?charge density?of the cation?(Group 2 metal ion) and the?polarisation??of?the?anion?(the nitrate and carbonate ion) attribute towards this increased stability
Trends in thermal stability going down the group
- All Group 2 metals form 2+ ions as they lose two electrons from their valence shells
- The metal cations at the top of the group are?smaller in size than those at the bottom
- For example, the atomic radius of beryllium (the first element in Group 2) is 112 pm whereas the atomic radius of calcium (further down the group) is 197 pm
- The metal cations at the top of Group 2, therefore, have the?greatest charge density?as the same charge (2+) is packed into a smaller volume
- As a result, smaller Group 2 ions have a?greater polarising effect?on neighbouring negative ions
- When a carbonate or nitrate ion approaches the cation, it becomes polarised
- This is because the metal cation draws the electrons in the carbonate or nitrate ion towards itself
- The?more polarised the anion?is, the?less heat is required?to thermally decompose them
- Therefore, the?thermal stability?increases down the group
- As down the group, the cation becomes larger
- Thus has a smaller charge density
- And a smaller polarising effect on the carbonate or nitrate anion
- So the anion is less polarised
- Therefore, more heat is required to thermally decompose them
Trends in Solubility & Enthalpy Change of Solution of Group 2 Hydroxides & Sulfates
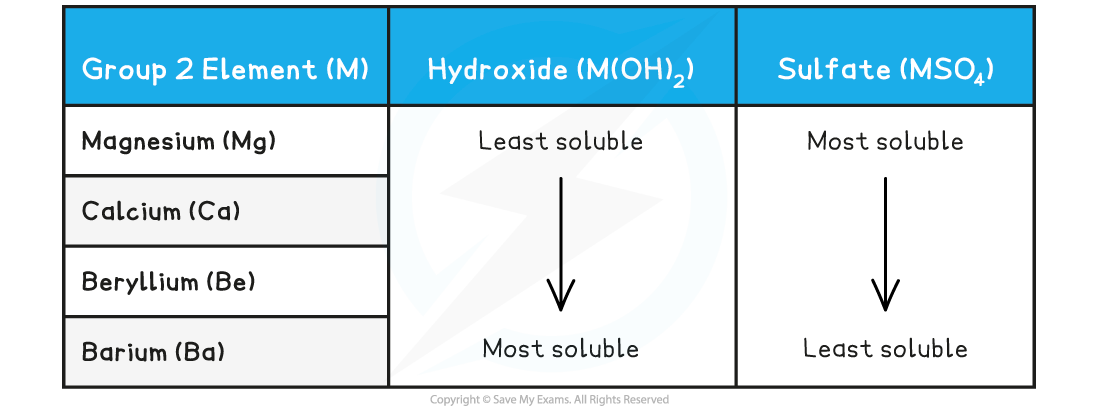
- The?solubility?of Group 2 hydroxides?increases?down the group
- In contrast, the Group 2 sulfates show a?decrease in solubility?going down the group
- Compounds that have very?low?solubility are said to be?sparingly soluble
- For example, calcium sulfate (CaSO4) has low solubility as only 0.21 g of CaSO4?dissolves in 100 g of water
- Most of the sulfates are soluble in warm water with the exception of?barium sulfate?which is?insoluble
Solubility of Group 2 elements table
Enthalpy change of hydration and lattice energy
- The?standard enthalpy of solution (ΔHsol?) is the energy?absorbed?or?released?when 1 mole of ionic solid dissolves in enough water to form a dilute solution (under standard conditions)
- The ΔHsol??can be either?exothermic?or?endothermic
- For example, the?ΔHsol??of sodium chloride (NaCl) is +3.9 kJ mol-1
NaCl (s) + aq → NaCl (aq)
OR
NaCl (s) + aq → Na+?(aq) + Cl-?(aq)
- This means, that 3.9 kJ mol-1?of energy is?absorbed?when one mole of NaCl is dissolved in enough water to form a dilute solution
- The?ΔHsol??is the sum of the?lattice energy?(ΔHlatt?) and the standard enthalpy change of hydration?(ΔHhyd?)
ΔHsol??=?ΔHlatt??+?ΔHhyd?
- The?lattice (formation) energy?is the energy?released?when?gaseous ions?combine to form?one mole?of an ionic compound under (standard conditions)
- Since energy is released when an ionic compound is formed, the?ΔHlatt??is always?exothermic
- For example, the?ΔHlatt??of NaCl is -787 kJ mol-1
Na+?(g) + Cl-?(g) → NaCl (s) ??
- This means, that 787 kJ mol-1?of energy is released when NaCl is formed from its gaseous ions
- The?standard enthalpy of hydration?is the?energy?released?when?gaseous ions?dissolve in enough water to form a dilute solution (under standard conditions)
- Since energy is released when gaseous ions become hydrated, the?ΔHhyd??is always?exothermic
- For example, the?ΔHhyd??of the sodium (Na+) ion is -406 kJ mol-1
Na+?(g) → Na+?(aq)
- This means, that 406 kJ mol-1?of energy is released when Na+?ions become hydrated
Trends of enthalpy change of solution
- Going down the group, the?ΔHlatt??of the ionic compounds?decreases
- Going down the group, the positively charged?cations?become larger
- There is more space between the negatively and positively charged ions in the ionic compound so there are weaker?attractive forces?between the ions
- As there are weaker?electrostatic forces?between the ions, there is less energy released upon formation of the ionic compound from its gaseous ions
- Therefore, the?ΔHlatt??becomes?less exothermic
- Going down the group, the?ΔHhyd??also?decreases
- Again, the positively charged ions become larger going down the group
- As a result, the?ion-dipole bonds?between the cations and water molecules get?weaker
- This means that?less energy?is released when the gaseous Group 2 ions become hydrated
- The?ΔHhyd??, therefore, becomes?less exothermic
- For?Group 2 hydroxides:
- Hydroxide ions are relatively small ions
- The?ΔHlatt??falls faster than the?ΔHhyd?
- The?enthalpy change of solution?is, therefore, more?exothermic?going down the group
- For?Group 2 sulfates:
- Sulfate ions are relatively large ions
- The?ΔHlatt??falls slower than the?ΔHhyd??enthalpy
- The?ΔHsol??will become more?endothermic?going down the group
- The more?exothermic?the?ΔHsol??the?more soluble?the compound
- This is why the?sulfates?become?less?soluble going down the group and the?hydroxides?more?soluble
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









