- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記5.6.6 Factors affecting Rate Constant
Effect of Temperature on the Rate Constant
- At higher temperatures, a greater proportion of molecules have energy greater than than the activation energy
- Since the?rate constant?and?rate of reaction?is?directly proportional?to the fraction of molecules with energy equal or greater than the activation energy, then at higher temperatures:
- The?rate constant?increases
- The?rate of reaction?increases
- The relationship between the rate constant and the temperature is given by the following equation:
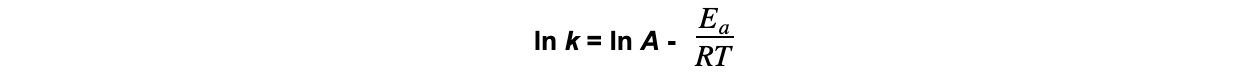
ln?k?= natural logarithm of the rate constant
A?= constant related to the collision frequency and orientation of the molecules
Ea?= activation energy (joules, J)
R?= gas constant (8.31 J K-1?mol-1)
T?= temperature (kelvin, K)
- A?varies only a little bit with temperature, it can be considered a constant
- Ea?and?R?are also constants
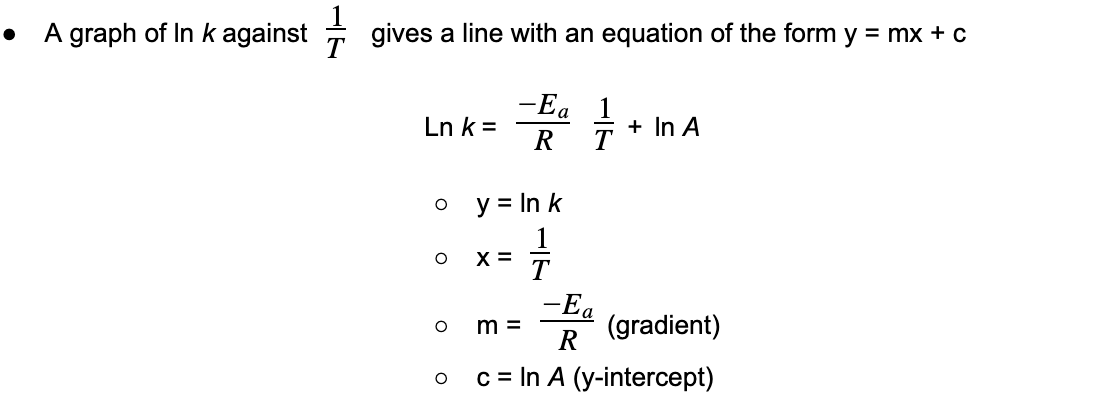
- The equation shows that an?increase?in temperature (higher value of?T) gives a?greater?value of ln?k?(and therefore a higher value of?k)
- Since the?rate of the reaction?depends on the?rate constant?(k) an increase in?k?also means an increased rate of reaction
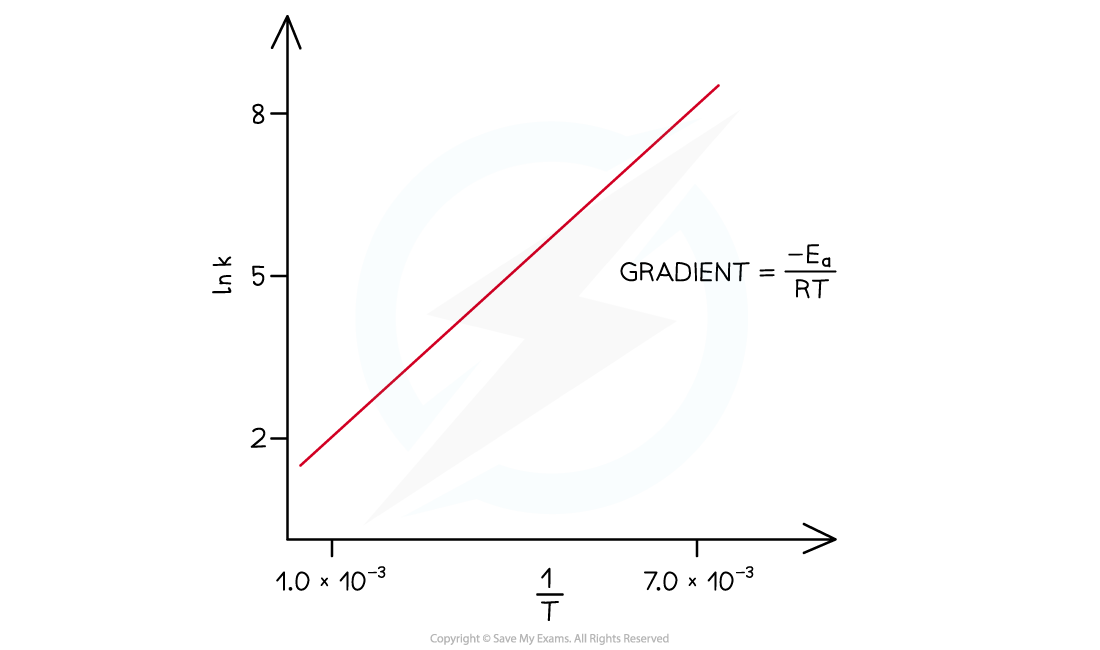
The graph of ln k over 1/T is a straight line with gradient -Ea/R
Exam Tip
You are not required to learn this equation however it is helpful in understanding the effects of temperature on the rate constant.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









