- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記2.1.2 Period 3 Elements: Structure & Bonding
Period 3: Structure & Bonding
Melting point
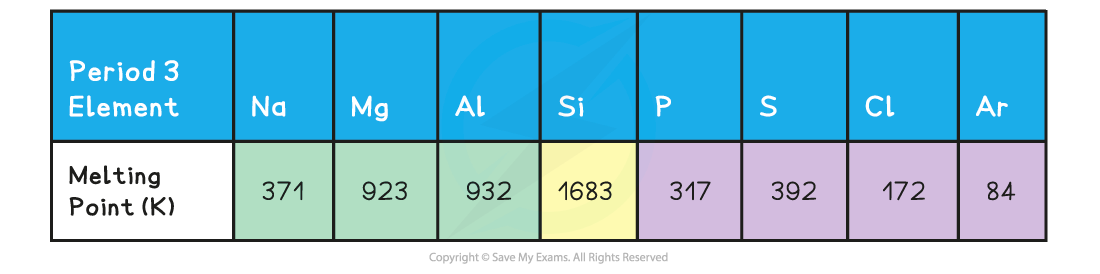
Melting points of the elements across Period 3 table
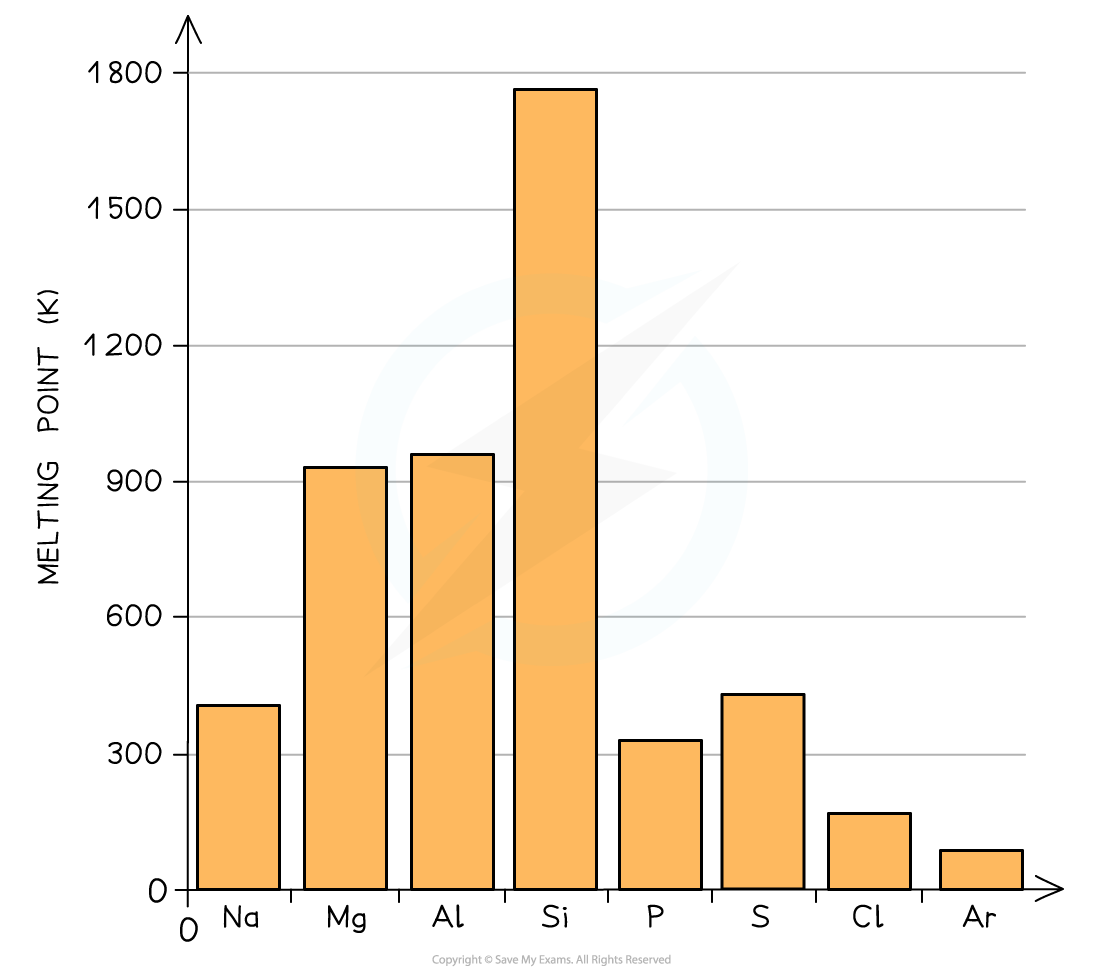
Ions of Period 3 elements with increasing positive charge (metals) and increasing of outer electrons across the period
- The above trends can be explained by looking at the bonding and structure of the elements
Bonding & structure of the elements table
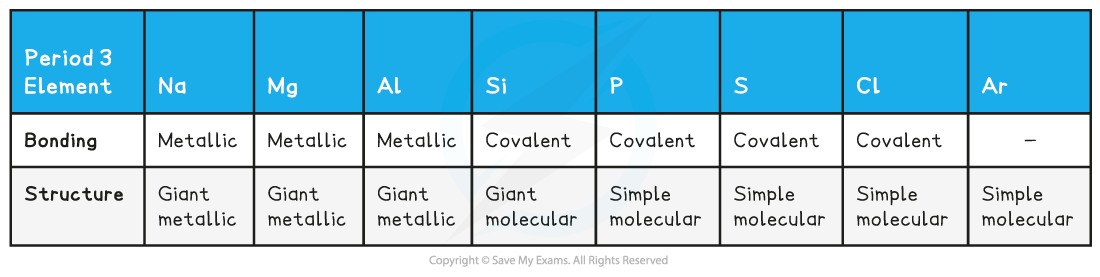
- The table shows that?Na,?Mg?and?Al?are metallic elements which form positive ions arranged in a?giant?lattice?in which the ions are held together by a 'sea' of?delocalised?electrons around them
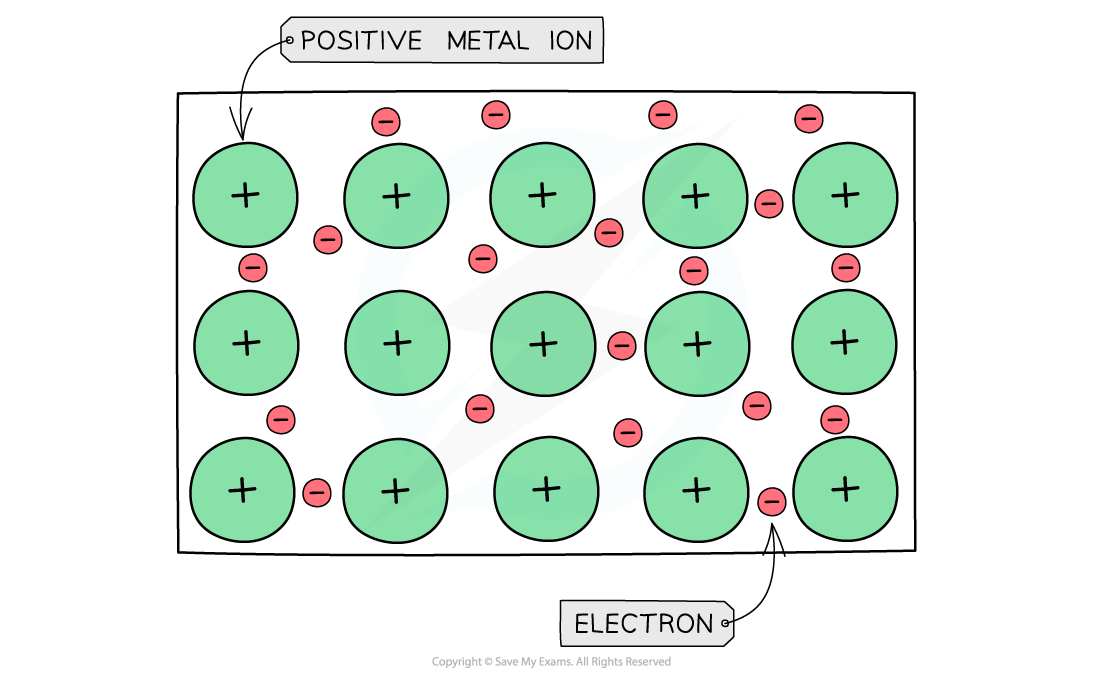
Metal cations form a giant lattice held together by electrons that can freely move around
- The electrons in the ‘sea’ of delocalised electrons are those from the?valence shell?of the atoms
- Na?will donate one electron into the ‘sea’ of delocalised electrons,?Mg?will donate two and?Al?three electrons
- As a result of this, the metallic bonding in?Al?is stronger than in?Na
- This is because the electrostatic forces between a?3+ ion?and the larger number of negatively charged delocalised electrons is much larger compared to a?1+ ion?and the smaller number of delocalised electrons in Na
- Because of this, the?melting points increase?going from?Na?to?Al
- Si?has the highest melting point due to its giant molecular structure in which each Si atom is held to its neighbouring Si atoms by?strong covalent bonds
- P, S,?Cl?and?Ar?are non-metallic elements and exist as?simple molecules?(P4, S8, Cl2?and Ar as a single atom)
- The?covalent bonds within?the molecules are strong, however,?between?the molecules, there are only weak?instantaneous dipole-induced dipole forces
- It doesn’t take much energy to break these?intermolecular?forces
- Therefore, the melting points decrease going from?P?to?Ar?(note that the melting point of S is higher than that of P as sulphur exists as larger S8?molecules compared to the smaller P4?molecule)
Electrical conductivity
- The?electrical conductivity decreases?going across the Period 3 elements
Electrical conductivity decreases Period 3 elements table
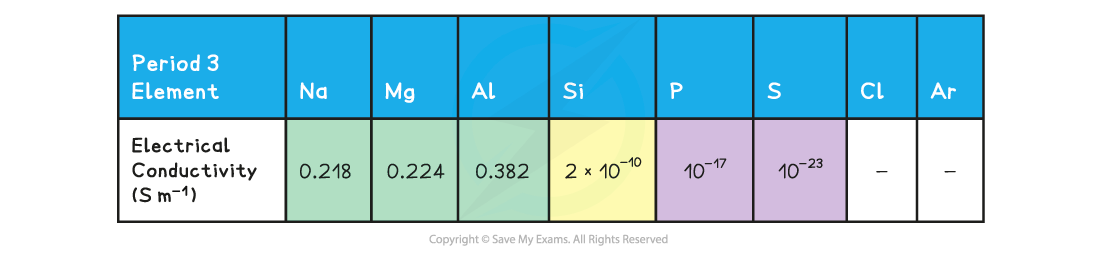
- Going from?Na?to?Al, there is an increase in the number of valence electrons that are donated to the ‘sea’ of delocalised electrons
- Because of this, in?Al?there are more electrons available to move around through the structure when it conducts electricity, making?Al?a better electrical conductor than?Na
- Due to the?giant molecular structure?of?Si, there are no delocalised electrons that can freely move around within the structure
- Si?is therefore not a good electrical conductor and is classified as a?semimetal?(metalloid)
- The lack of delocalised electrons is also why?P?and?S?cannot conduct electricity
Exam Tip
Intermolecular forces?are forces?between?moleculesIntramolecular forces?are forces?within?a molecule
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









