- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記1.3.1 Electronegativity
Electronegativity: Definition
- Electronegativity?is the ability of an atom to attract a pair of electrons towards itself in a covalent bond
- This phenomenon arises from the?positive?nucleus’s ability to attract the?negatively?charged electrons, in the outer shells, towards itself
- The?Pauling?scale?is used to assign a value of electronegativity for each atom
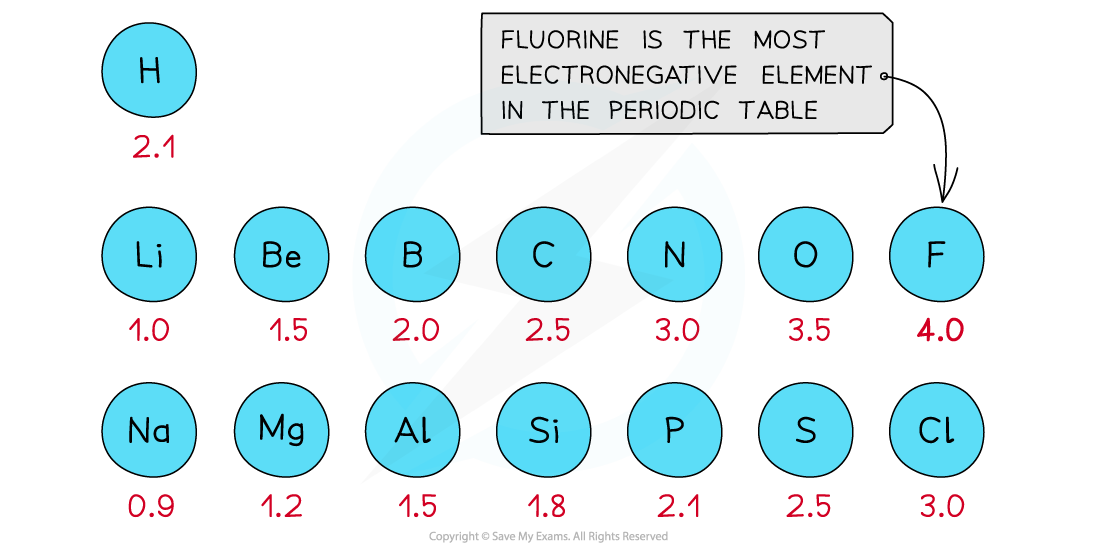
First three rows of the periodic table showing electronegativity values
- Fluorine is the most electronegative atom on the Periodic Table, with a value of 4.0 on the?Pauling Scale
- It is best at attracting electron density towards itself when covalently bonded to another atom
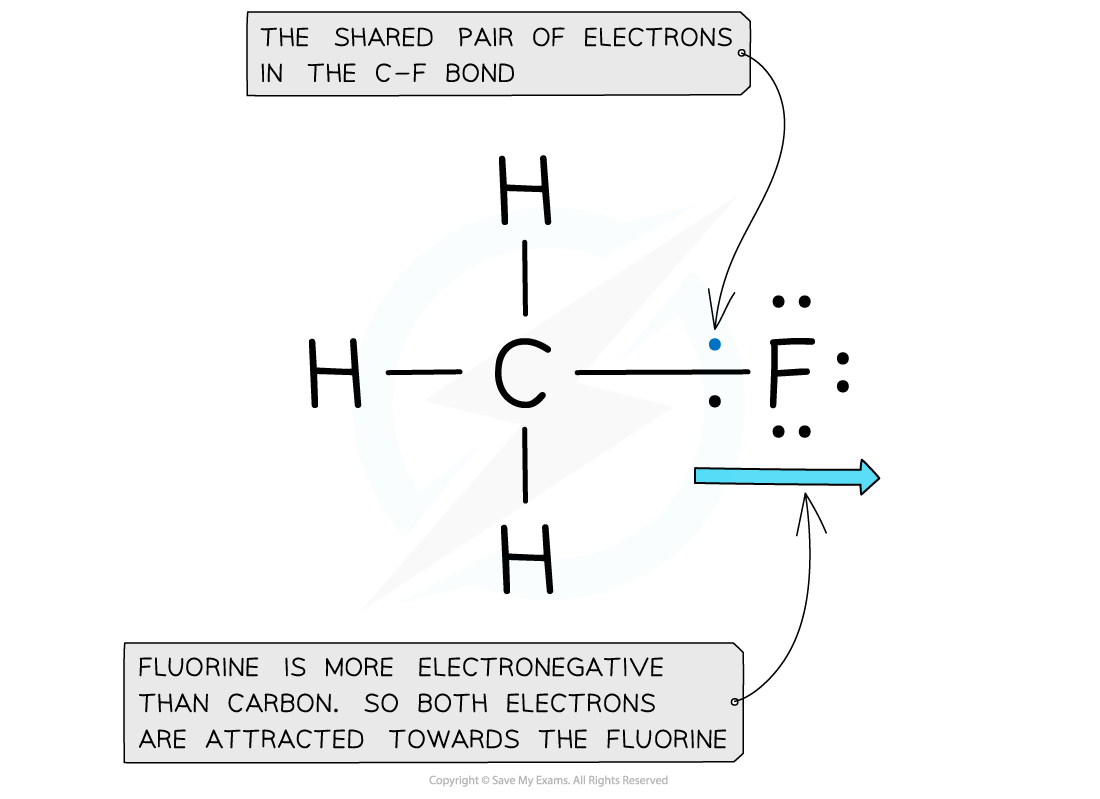
Electron distribution in the C-F bond of fluoromethane
Electronegativity: Affecting Factors
Nuclear charge
- Attraction?exists between the positively charged?protons?in the nucleus and negatively charged?electrons?found in the energy levels of an atom
- An?increase?in the number of?protons?leads to an?increase?in?nuclear?attraction?for the electrons in the outer shells
- Therefore, an?increased nuclear charge?results in an?increased electronegativity
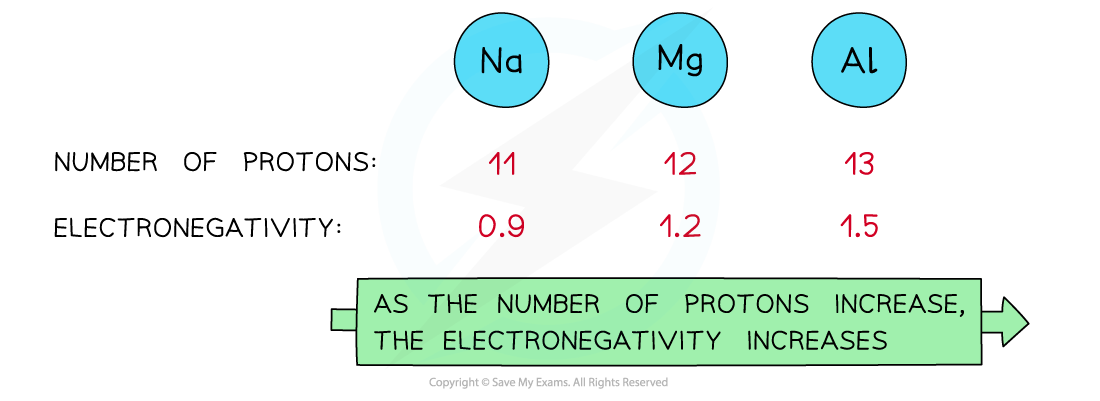
As the nuclear charge increases, the electronegativity of an element increases as well
Atomic radius
- The?atomic?radius?is the distance between the nucleus and electrons in the?outermost shell
- Electrons?closer?to the nucleus are more?strongly?attracted towards its positive?nucleus
- Those electrons?further?away?from the nucleus are?less strongly?attracted towards the?nucleus
- Therefore, an?increased atomic radius?results in a?decreased electronegativity
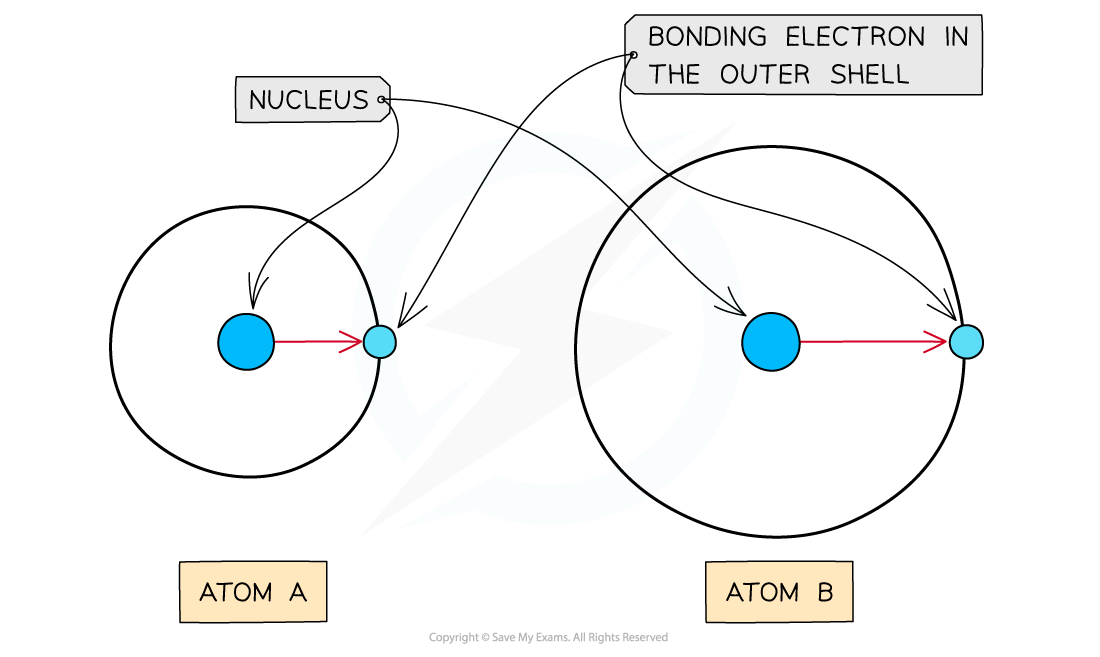
As the atomic radius increases, the nucleus has less of an attraction for the bonding electrons causing atom A to have a higher electronegativity than atom B
Shielding
- Filled?energy levels can?shield?(mask) the effect of the nuclear charge causing the outer electrons to be?less attracted?to the nucleus
- Therefore, the addition of extra?shells and subshells?in an atom will cause the outer electrons to experience?less?of the attractive force of the nucleus
- Sodium (Period 3, Group 1) has higher?electronegativity?than caesium (Period 6, Group 1) as it has fewer shells and therefore the outer electrons experience less shielding than in caesium
- Thus, an increased number of?inner shells and subshells?will result in a?decreased electronegativity
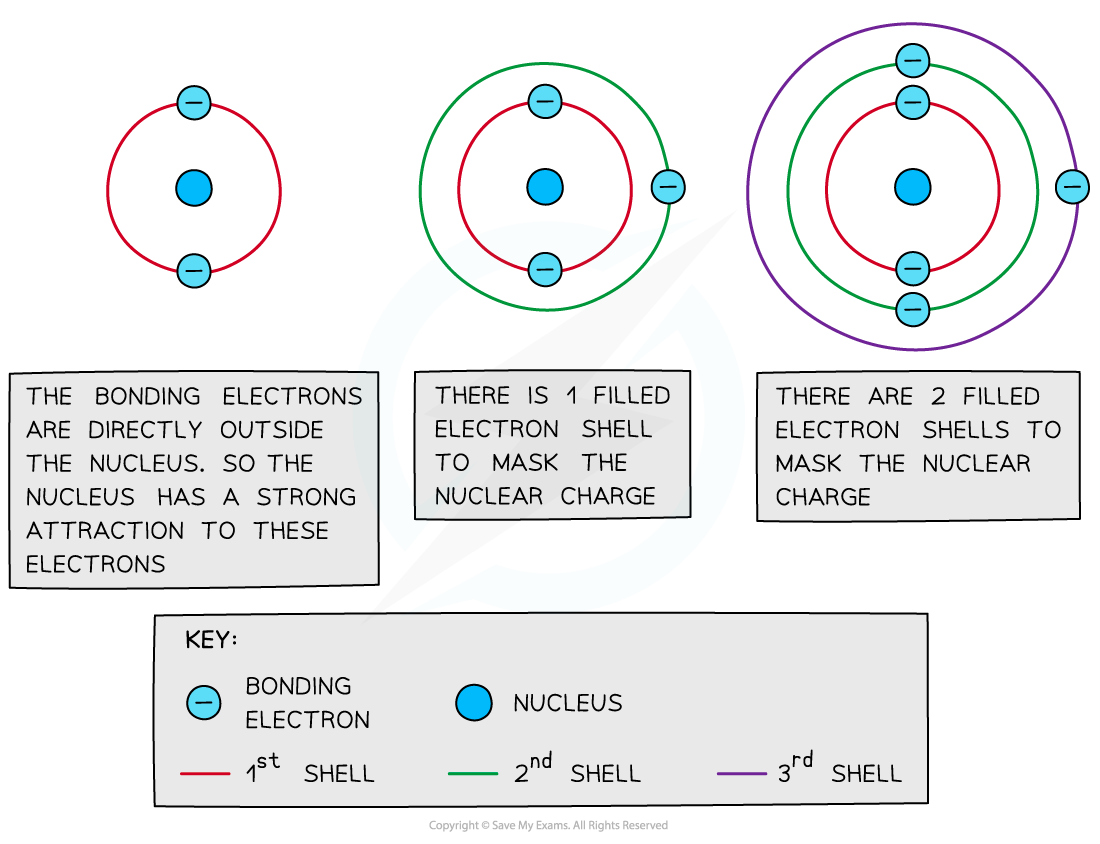
Filled inner energy levels mask the nuclear attraction from the outer bonding electrons
Exam Tip
The?nuclear charge,?atomic radius?and?shielding?are all linked to each other.As?nuclear?charge?increases, the nucleus has a?greater?attractive?force?on the electrons in shells given that the?shielding?doesn’t increase.As a result of this, the atomic radius decreases.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









