- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Chemistry復習筆記1.1.11 Ionisation Energy Trends
Ionisation Energies: Trends
- Ionisation energies show?periodicity?- a trend across a period of the Periodic Table
- As could be expected from their electronic configuration, the group I metals have a relatively low ionisation energy, whereas the noble gases have very high ionisation energies
- The size of the first ionisation energy is affected by four factors:
- Size of the nuclear charge
- The nuclear charge increases with increasing atomic number, which means that there are greater?attractive?forces?between the nucleus and electrons, so more energy is required to overcome these attractive forces when removing an electron
- Distance of outer electrons from the nucleus
- Electrons in shells that are further away from the nucleus are less attracted to the nucleus - the nuclear attraction is weaker - so the further the outer electron shell is from the nucleus, the?lower?the ionisation energy
- Shielding effect of inner electrons
- The?shielding effect?is when the electrons in full inner shells repel electrons in outer shells, preventing them from feeling the?full nuclear charge,?so the more shells an atom has, the greater the shielding effect, and the lower the ionisation energy
- Spin-pair repulsion
- Electrons in the same atomic orbital in a subshell repel each other more than electrons in different atomic orbitals which makes it easier to remove an electron (which is why the first ionization energy is always the lowest)
- Size of the nuclear charge
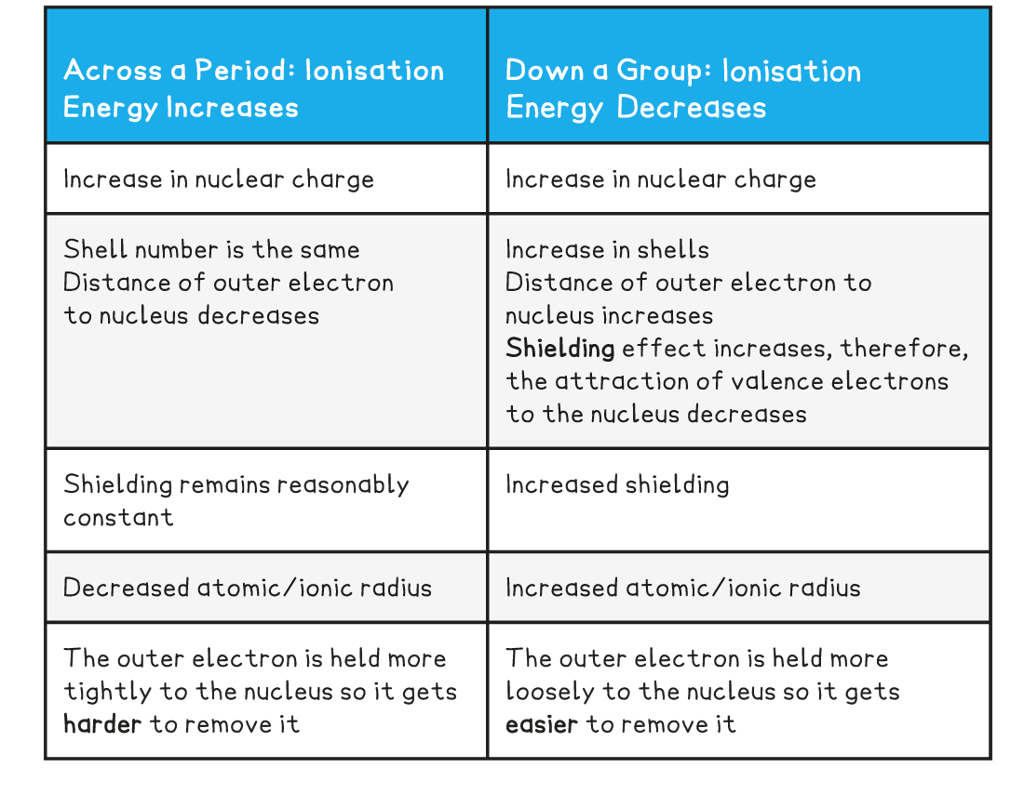
- So, the?first?ionisation energy?increases?across a period and?decreases?down a group

A graph showing the ionisation energies of the elements hydrogen to sodium
Ionisation energy across a period
- The ionisation energy over a period?increases?due to the following factors:
- Across a period the?nuclear charge increases
- This causes the?atomic radius?of the atoms to?decrease,?as the outer shell is pulled closer to the nucleus, so the distance between the nucleus and the outer electrons?decreases
- The?shielding?by inner shell electrons remain reasonably constant as electrons are being added to the same shell
- It becomes?harder to remove an electron?as you move across a period;?more energy?is needed
- So, the ionisation energy increases
- There is a rapid?decrease?in ionisation energy between the?last?element in one period, and the?first?element in the next period because:
- There is increased?distance?between the nucleus and the outer electrons as you have added a new shell
- There is increased?shielding?by inner electrons because of the added shell
- These two factors outweigh the increased?nuclear?charge
- There is a slight?decrease?in IE1?between?beryllium?and?boron?as the fifth electron in boron is in the 2p subshell, which is further away from the nucleus than the 2s subshell of beryllium
- Beryllium?has a first ionisation energy of?900 kJ mol-1?as its electron configuration is?1s2?2s2
- Boron?has a first ionisation energy of?800 kJ mol-1?as its electron configuration is?1s2?2s2?2px1
- There is a slight?decrease?in IE1?between?nitrogen?and?oxygen?and?phosphorus?due to?spin-pair repulsion?in the 2px?orbital of oxygen
- Nitrogen?has a first ionisation energy of?1400 kJ mol-1?as its electron configuration is 1s2?2s2?2px1?2py1?2pz1
- Oxygen?has a first ionisation energy of?1310 kJ mol-1?as its electron configuration is 1s2?2s2?2px2?2py1?2pz1
Ionisation energy down a group
- The ionisation energy down a group?decreases?due to the following factors:
- The number of protons in the atom is increased, so the?nuclear charge?increases
- But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger
- So, the?distance?between the nucleus and outer electron?increases?as you descend the group
- The?shielding?by inner shell electrons?increases?as there are more shells of electrons
- These factors outweigh the increased nuclear charge, meaning it becomes?easier to remove the outer electron?as you descend a group
- So, the ionisation energy decreases
Ionisation energy trends across a period & going down a group table
Successive ionisation energies of an element
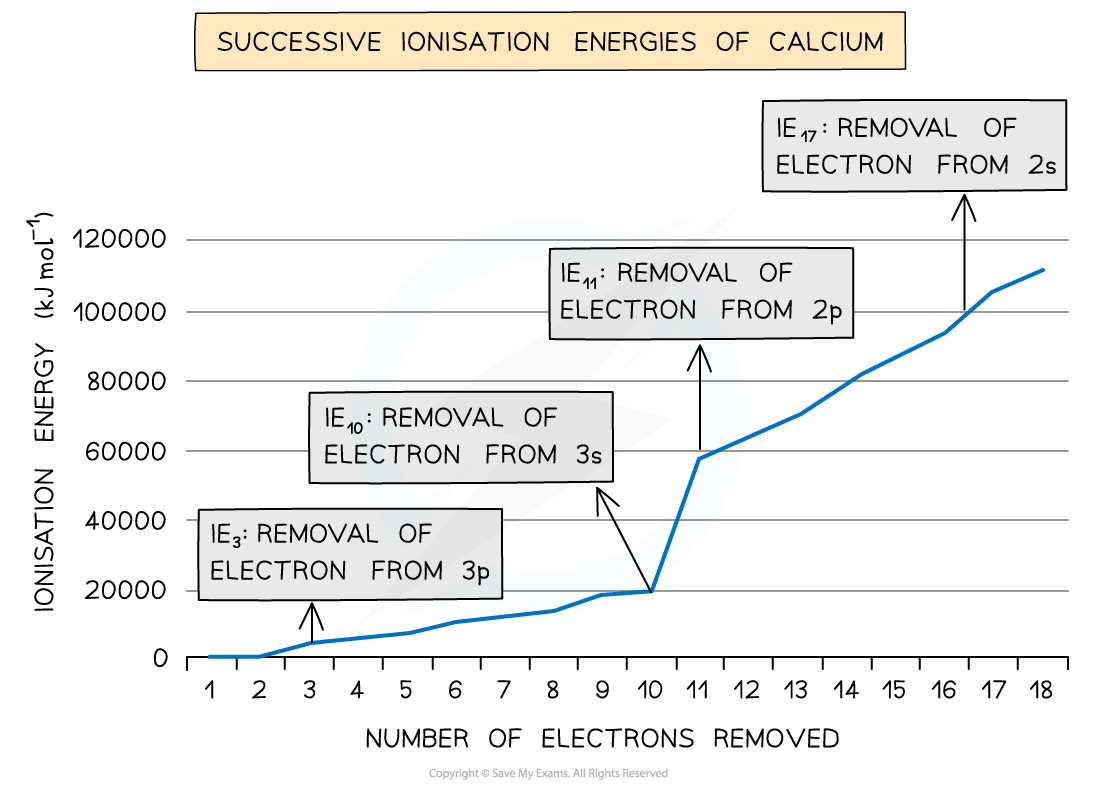
- The?successive?ionisation energies of an element?increase
- This is because once you have removed the outer electron from an atom, you have formed a?positive ion
- Removing an electron from a positive ion is?more difficult?than from a neutral atom
- As more electrons are removed, the?attractive forces increase?due to decreasing shielding and an increase in the proton to electron ratio
- The increase in ionisation energy, however, is not constant and is dependent on the atom's electronic configuration
- Taking calcium as an example:
Ionisation energies of calcium table
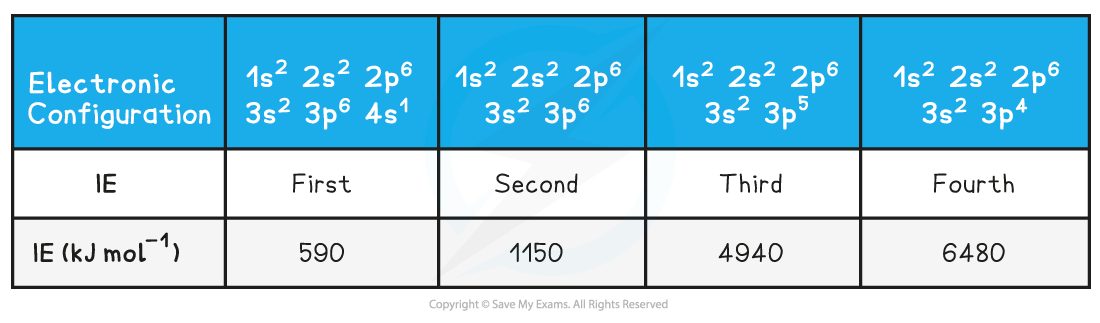
- The?first?electron removed has a low IE1?as it is easily removed from the atom due to the spin-pair repulsion of the electrons in the 4s orbital
- The?second?electron is more difficult to remove than the first electron as there is no?spin-pair repulsion
- The?third?electron is much more difficult to remove than the second one corresponding to the fact that the third electron is in a?principal quantum?shell which is closer to the nucleus (3p)
- Removal of the?fourth?electron is more difficult as the orbital is no longer full, and there is less?spin-pair repulsion
Exam Tip
It is easy to remove electrons from a full subshell as they undergo?spin-pair repulsion.It gets more difficult to remove electrons from?principal quantum shells?that get closer to the nucleus as there is less?shielding?and an increase in?attractive forces?between the electrons and nuclear charge.
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









