- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
CIE A Level Biology復習筆記3.1.4 Measuring Enzyme Activity
Measuring Enzyme Activity
- The?progress?of?enzyme-catalysed reactions?can be investigated by:
- Measuring the?rate of formation of a product?using catalase
- Measuring the?rate of disappearance of a substrate?using amylase
Investigating catalase activity
- In this investigation, the rate of?product formation?is used to measure the rate of an enzyme-controlled reaction:
- Hydrogen peroxide?is a common but?toxic?by-product of metabolism
- This means it must be?broken down?quickly
- Catalase?is an enzyme found in the cells of most organisms that?breaks hydrogen peroxide down into water and oxygen
- Hydrogen peroxide and catalase are combined and the?volume of oxygen generated?is measured in a set time
- The?rate of reaction?can then be calculated
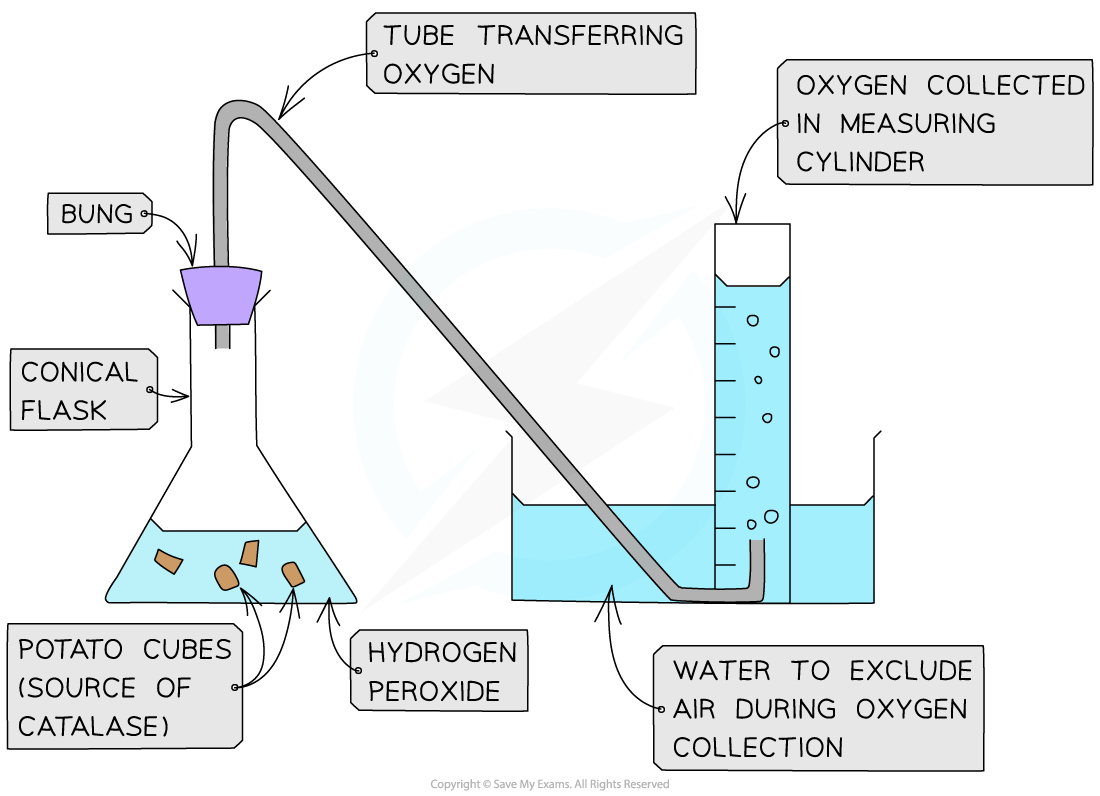
Experimental set-up for investigating the rate of formation of a product using catalase
Investigating amylase activity
- In this investigation, the rate of?substrate disappearance?is used to compare rates of reaction under different conditions:
- Amylase?is a digestive enzyme that?hydrolyses starch into maltose and glucose
- Amylase functions best at pH 7 and 37oC (all enzymes operate best under specific conditions)
- Amylase and starch are combined?and this reaction mixture is then?tested for starch?at regular time intervals
- This can be done by taking samples from the reaction mixture at each time interval and adding each sample to some?iodine in potassium iodide solution?(starch forms a?blue-black?colour with this solution)
- In this way, the time taken for starch to be broken down can be measured
- The investigation can be repeated under a variety of conditions?(eg. by altering pH or temperature) and the?reaction rates can then be compared
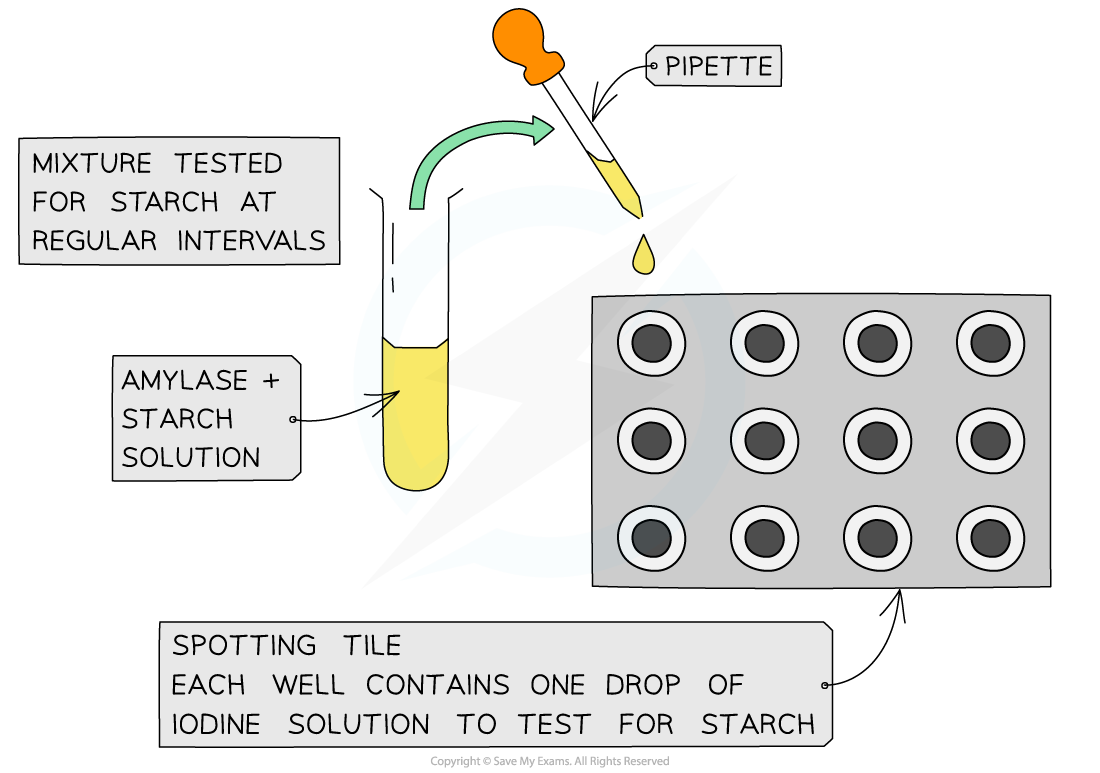
Experimental set-up for investigating the rate of disappearance of a substrate using amylase
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









