- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Chemistry: SL復習筆記8.2.2 pH & [H?]
pH & [H?]
- The acidity of an aqueous solution depends on the number of?H+?(H3O+) ions in solution
- The?pH?is defined as:

-
- where [H+] is the concentration of H+?in mol dm–3
- The pH scale is a logarithmic scale with base 10
- This means that each value is 10 times the value below it. For example, pH 5 is 10 times more acidic than pH 6.
- pH values are usually given to 2 decimal places
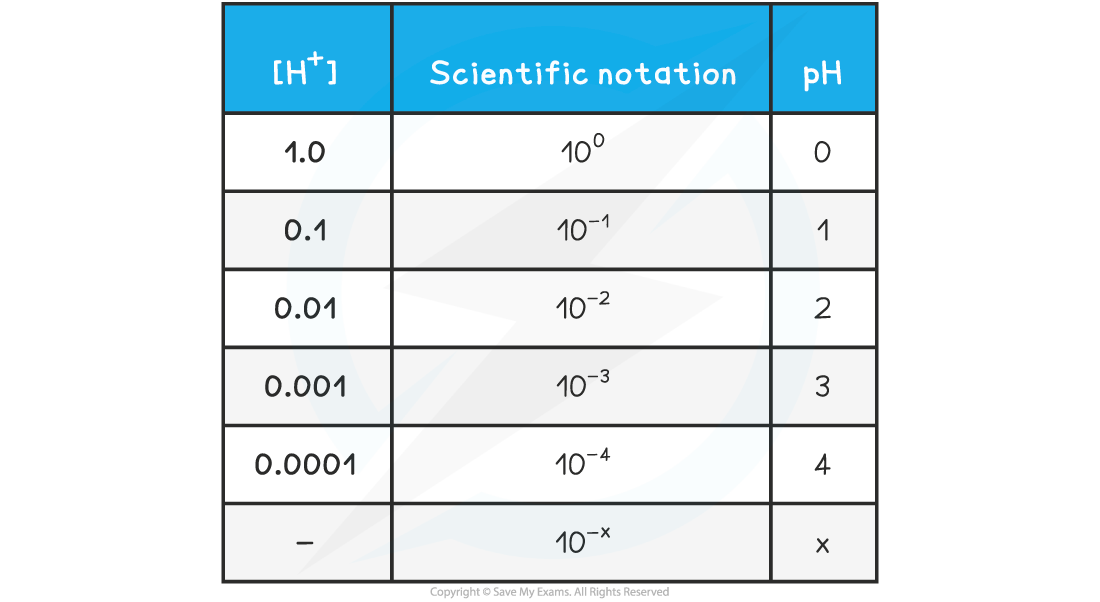
- The relationship between concentration is easily seen on the following table:
pH & [H+] Table
Worked Example
10.0 cm3?of an aqueous solution of nitric acid of pH = 1.0 is mixed with 990.0 cm3?of distilled water. What is the pH of the final solution?
A.??1
B.??2
C.??3
D.??10
Answer:
The correct option is?C.
-
- The total volume after dilution is 1000.0 cm3?so the concentration of H+?has been?reduced?by a factor of 100 or 10-2, which means an increase of 2 pH units
- The final solution is therefore?pH 3
Exam Tip
Make sure you know how to use the antilog (base 10) feature on your calculator. On most calculators it is the 10x?button, but on other models it could be LOG-1, ALOG or even a two-button sequence such as INV + LOG
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









