- 翰林提供學(xué)術(shù)活動(dòng)、國(guó)際課程、科研項(xiàng)目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
IB DP Chemistry: SL復(fù)習(xí)筆記8.1.1 Br?nsted–Lowry Acids & Bases
Br?nsted–Lowry Acids & Bases
- The Br?nsted-Lowry Theory?defines acids and bases in terms of proton transfer between chemical compounds
- A?Br?nsted-Lowry acid?is a species that?gives away?a?proton?(H+)
- A?Br?nsted-Lowry base?is a species that?accepts?a proton (H+) using its?lone pair of electrons
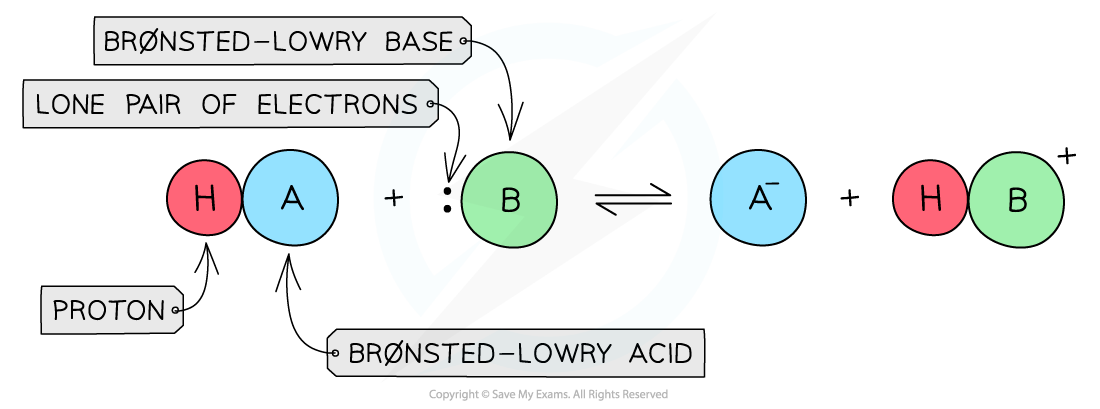
The diagram shows a Br?nsted-Lowry acid which donates the proton to the Br?nsted-Lowry base that accepts the proton using its lone pair of electrons
- The Br?nsted-Lowry Theory is not limited to aqueous solutions only and can also be applied to reactions that occur in the gas phase
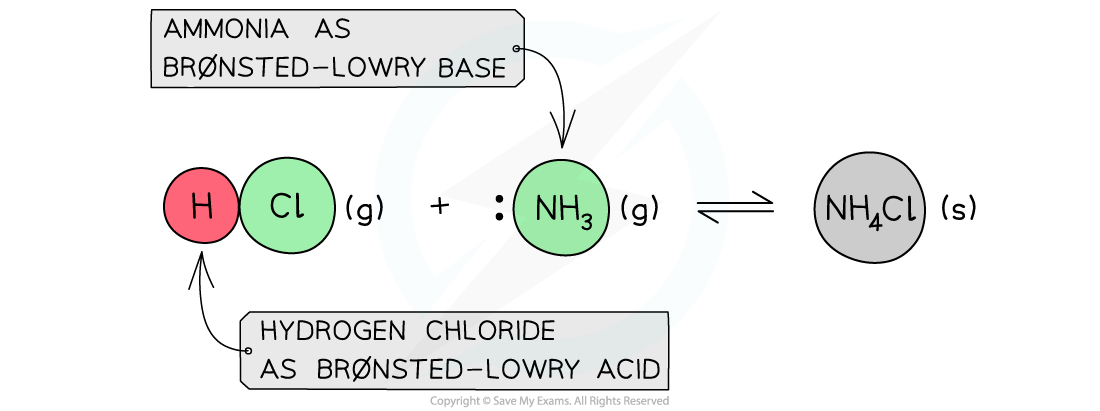
Example of a Br?nsted-Lowry acid and base reaction in the gas state
Worked Example
Identify the correct role of the species in the following reaction:
H2PO4?(aq) + H2O(l) → HPO42?(aq) + H3O+(aq)
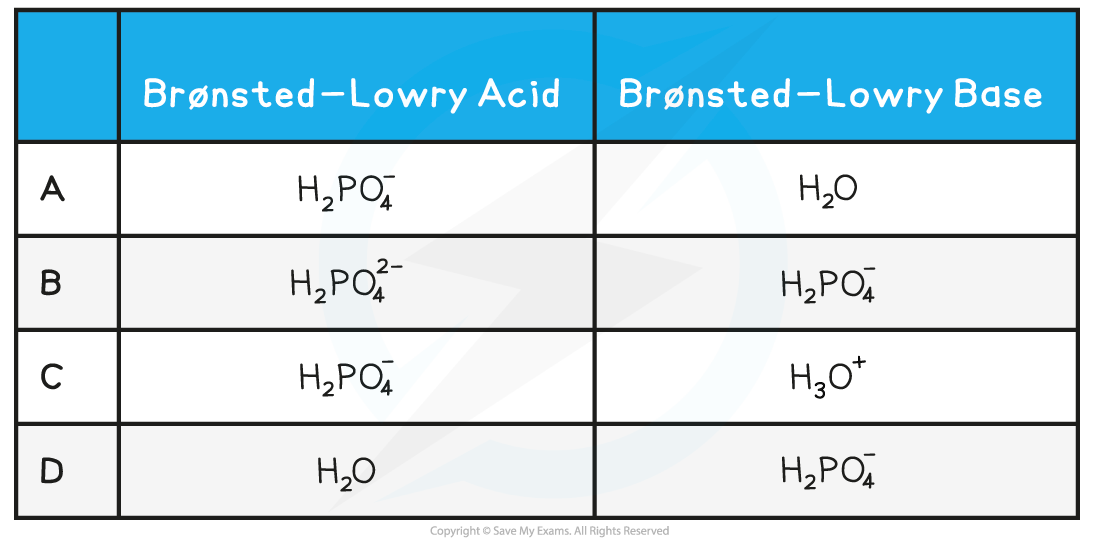
Answer:
The correct option is?A.
-
- H2PO4??is donating a proton to H2O, so H2PO4??must be an acid and H2O must be a base
Exam Tip
An atom of hydrogen contains 1?proton, 1 electron and 0 neutrons. When hydrogen loses an electron to become?H+?only a?proton?remains, which?is why a H+?ion is also called a proton.
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號(hào)-1









