- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IB DP Chemistry: SL復習筆記1.2.2 Reaction Yields
Reaction Yields
Percentage yield
- In a lot of reactions, not all reactants react to form products which can be due to several factors:
- Other reactions take place simultaneously
- The reaction does not go to?completion
- Products are?lost?during separation and purification
- The?percentage yield?shows how much of a particular product you get from the reactants compared to the maximum theoretical amount that you can get:
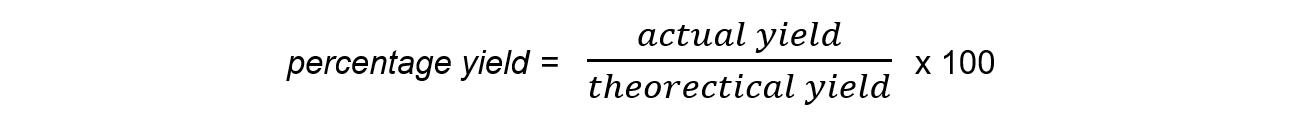
- The?actual yield?is the number of moles or mass of product obtained?experimentally
- The?theoretical?yield?is the number of moles or mass obtained by a reacting mass calculation
Worked Example
In an experiment to displace copper from copper(II)sulfate, 6.5 g of zinc was added to an excess of copper(II)sulfate solution.The resulting copper was filtered off, washed and dried.The mass of copper obtained was 4.8 g.Calculate the percentage yield of copper.
Answer:
Step 1:?The symbol equation is:
Zn (s)+CuSO4 (aq)→ZnSO4 (aq)+ Cu (s)
Step 2:?Calculate the amount of zinc reacted in moles
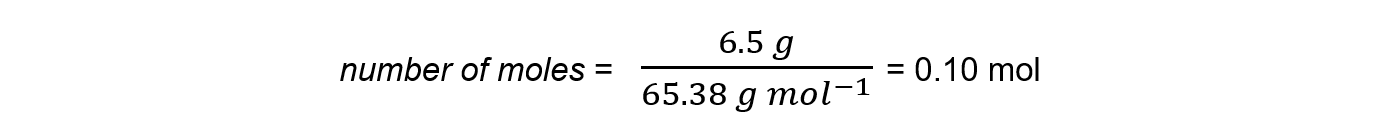
Step 3:?Calculate the maximum amount of copper that could be formed from the molar ratio:
Since the ratio of Zn(s) to Cu(s) is 1:1 a maximum of 0.10 moles can be produced
Step 4:?Calculate the maximum mass of copper that could be formed (theoretical yield)
mass=molxM=0.10molx63.55g mol-1=6.4 g (2 sig figs)
Step 5:?Calculate the percentage yield of copper
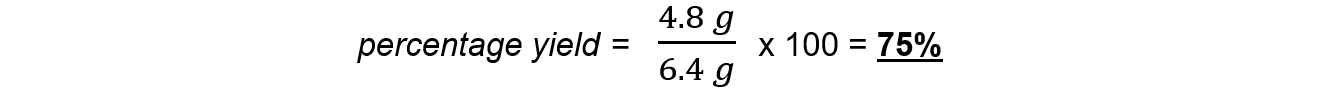
轉載自savemyexams

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









