Diagram shows that a catalyst increases the rate of a reaction by providing an alternative pathway which has a lower activation energy
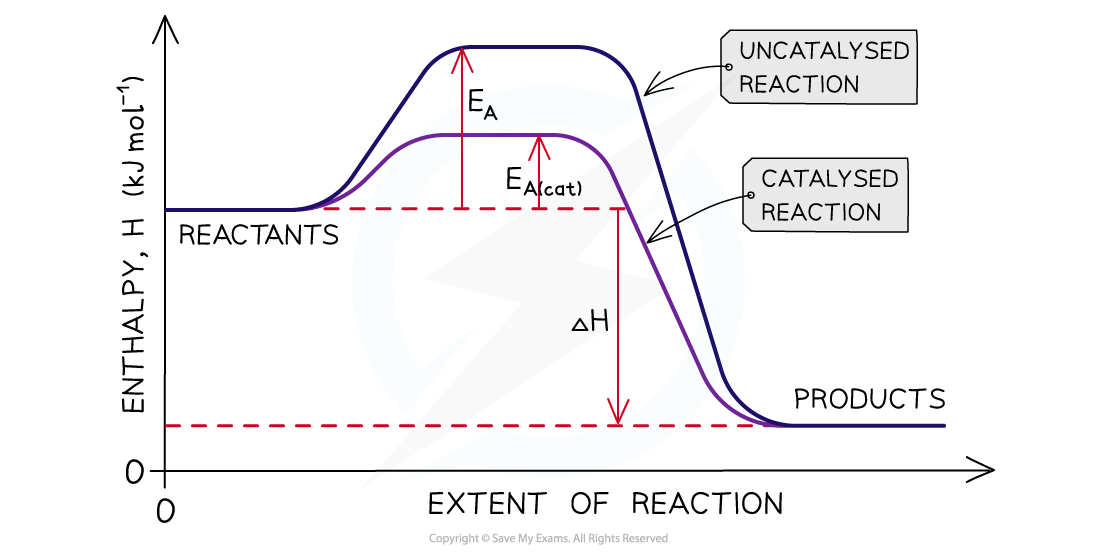
Diagram shows that a catalyst increases the rate of a reaction by providing an alternative pathway which has a lower activation energy
The activation energy is constant for a particular reaction. Reactions with a low activation energy occur readily as little energy is needed to break the bonds and initiate the reaction.
轉載自savemyexams

? 2025. All Rights Reserved. 滬ICP備2023009024號-1