- 翰林提供學(xué)術(shù)活動、國際課程、科研項目一站式留學(xué)背景提升服務(wù)!
- 400 888 0080
AQA A Level Chemistry復(fù)習(xí)筆記 6.1.4 Oxides Reacting with Water
Oxides Reacting with Water
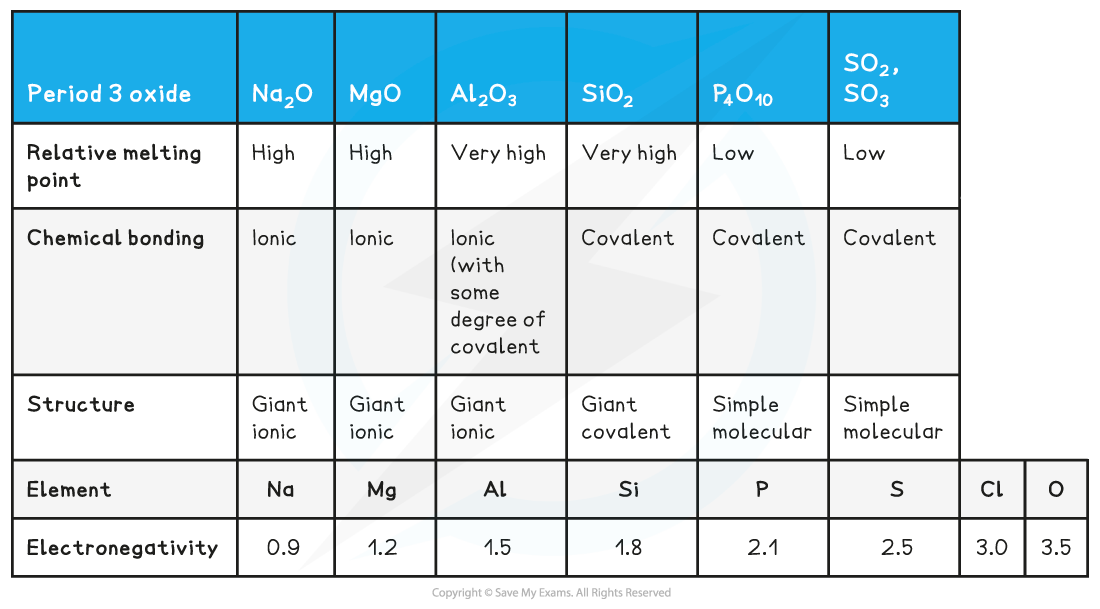
Structure, bonding & electronegativity of the Period 3 elements table
- The oxides of?Na and Mg?which show purely?ionic bonding?produce?alkaline?solutions with water as their?oxide?ions (O2-) become?hydroxide?ions (OH-):
O2-?(aq) + H2O (l) → 2OH-?(aq)
- The oxides of?P and S?which show purely?covalent bonding?produce?acidic?solutions with water because when these oxides react with water, they form an acid which donates?H+?ions to water
- Eg. SO3?reacts with water as follows:
SO3?(g) + H2O (l) → H2SO4?(aq)
-
- The H2SO4?is an acid which will donate a H+?to water:
H2SO4?(aq) + H2O (l) → H3O+?(aq) + HSO4-?(aq)
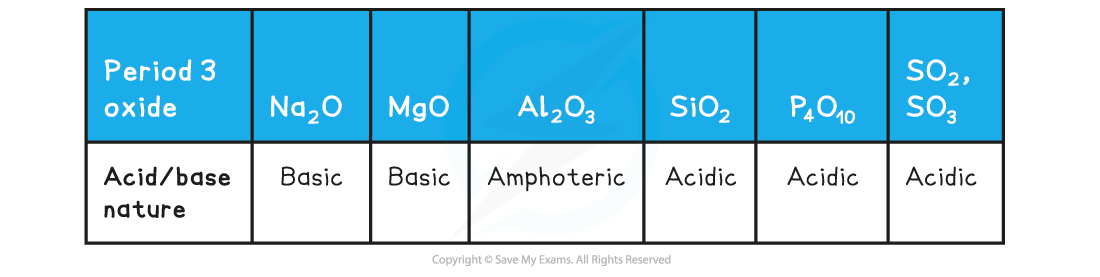
- Al?and?Si?are insoluble and when they react with?hot, concentrated alkaline solution?they act as a base and form a salt
- This behaviour is very typical of a?covalently bonded oxide
- Al?can also react with?acidic solutions?to form a salt and water
- This behaviour is very typical of an?ionic bonded metal oxide
- This behaviour of?Al?proves that the chemical bonding in aluminium oxide is not purely?ionic?nor?covalent: therefore it exhibits amphoteric character
Reaction of Period 3 oxides with water table
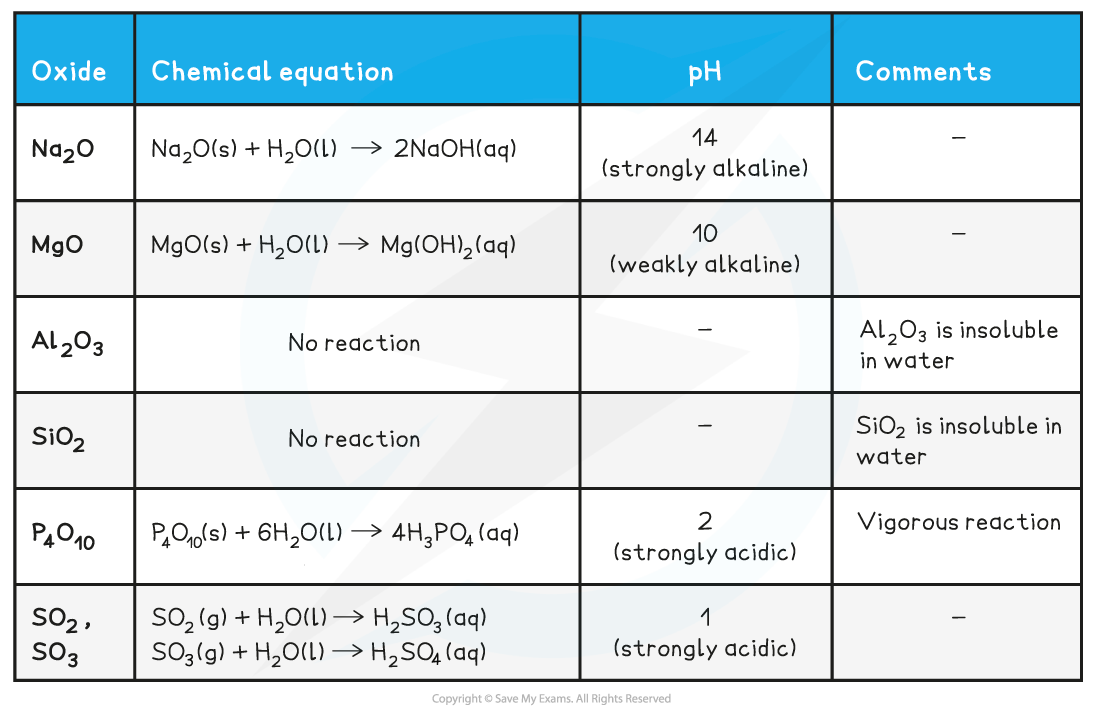
Behaviour of the Period 3 Oxides with Water
- Metal oxides (to the left of the periodic table):
- Sodium oxide, Na2O, and magnesium oxide, MgO, are made up of ions
- They contain an oxide ion, O2-, which is a strong base and will readily produce hydroxide ions through reaction with water
- This is why the solutions formed are strongly alkaline
- Sodium oxide forms a more alkaline solution than magnesium oxide because it is far more soluble in water
- Oxides in the middle of the periodic table
- Although ionic, aluminium oxide does not react with water because the oxide ions are held too strongly in the ionic lattice
- This means the ions cannot be separated
- Silicon dioxide is a giant covalent molecule - it is the main component of sand
- It has millions of strong covalent bonds, so it does not react with water
- Non-metal oxides (to the right of the periodic table):
- Oxides of phosphorus and sulfur are simple covalent molecules
- They will react with water to produce acidic solutions
Exam Tip
Key thing to remember: The metal oxides form alkaline solutions in water, the oxides in the middle do not react and the non-metal oxides form acidic solutions.
Acid-Base Reactions of the Oxides
Acid/base Nature of the Period 3 Oxides
- Aluminium oxide is?amphoteric?which means that it can act both as a base (and react with an acid such as HCl) and an acid (and react with a base such as NaOH)
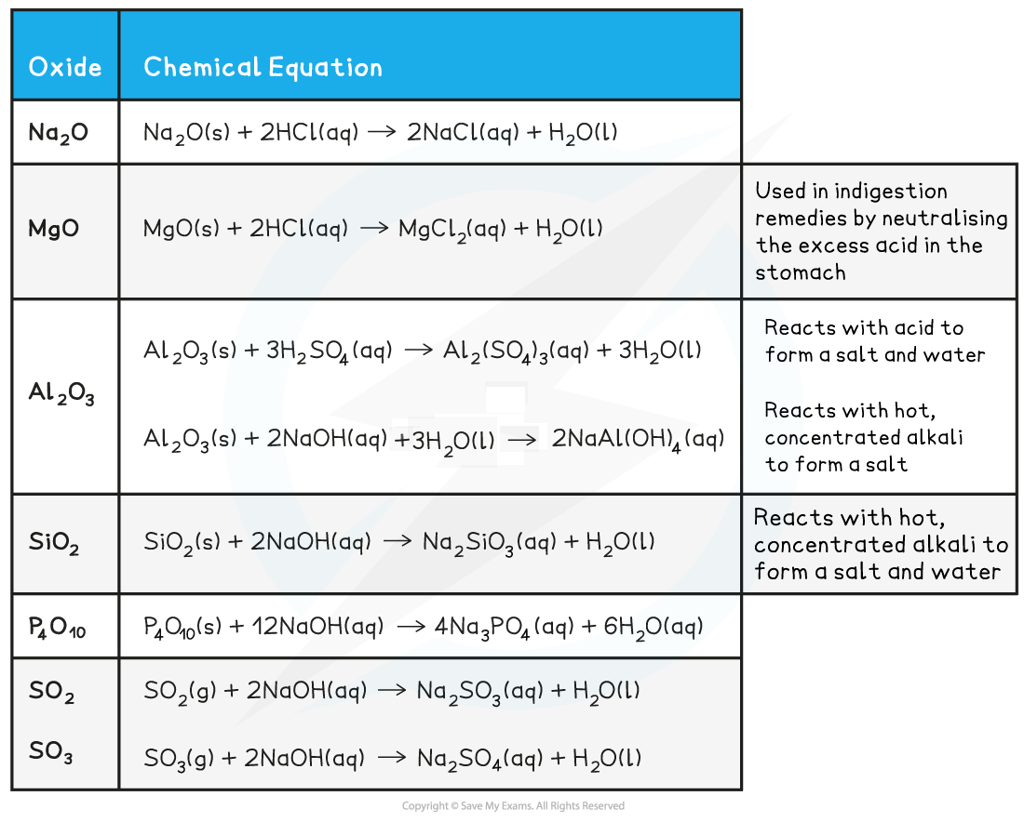
Reactions of the Period 3 oxides with acid/base table
Exam Tip
It is crucial that you learn these reactions - make sure that you know the state symbols, the products formed and the full balanced equations!
轉(zhuǎn)載自savemyexams

最新發(fā)布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









