- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IBDP化學課程真題講解(1)
通常而言,IBDP 化學被視作一門比較難的學科。但對于一些小伙伴而言,IB化學卻并不那么難。這兩者并不矛盾。今天帶著大家一起看一道IBDP化學課程的真題:選擇題(HIGHER LEVEL PAPER 1)。這道題取自于IBDP chemistry specimen paper。
題目1:Which statement about transition metal complex ions is correct?
A. The difference in energy of the d orbitals is independent of the oxidation state of the metal.
B. The colour of the complex is caused by light emitted when an electron falls back from a higher to a lower energy level.
C. The colour of the complex is the colour of the light absorbed when an electron moves from a lower to a higher energy level.
D. The difference in energy of the d orbitals depends on the nature of the ligand.
題目難度:簡單。
考點分析:考查complex ion
題目分析:A選項oxidation state of the metal是會影響differencein energy of the d orbitals的,所以錯誤;B選項是electron被excited到higher energy level所吸收的光的補色,即為complex的顏色,所以錯誤;C選項同上,錯誤;D選項正確。
正確答案:D
題目2:Which element is in group 2?
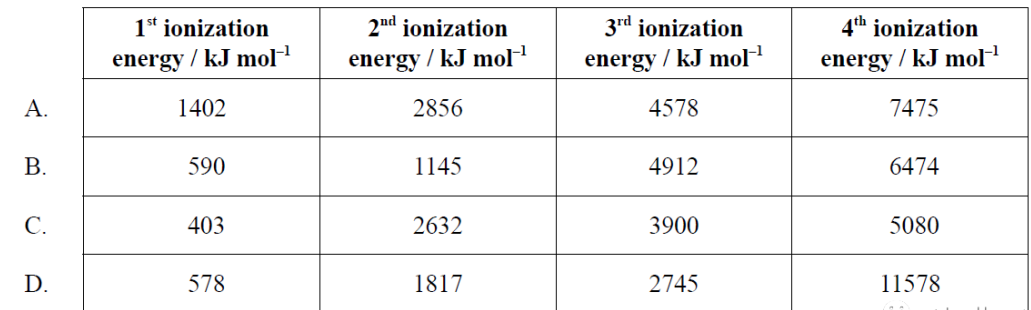
題目難度:簡單。
考點分析:考查group 2的ionization energy
題目分析:group 2最外層有2個電子,所以前2個電子失去的時候所需能量相差不大,但是第2個和第3個相差較大,B選項的第2個數和第3個數相差較大,所以是屬于group 2的element,所以答案選B。
正確答案:B
掃碼添加翰林顧問老師,可一對一制定國際課程規劃
【免費領取】IBDP/IB 備考資料合集~


最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









