- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
IBDP化學課程真題講解(2)
通常而言,IBDP 化學被視作一門比較難的學科。但對于一些小伙伴而言,IB化學卻并不那么難。這兩者并不矛盾。今天帶著大家一起看一道IBDP化學課程的真題講解(HIGHER LEVEL PAPER 2)。這道題取自于IBDP chemistry specimen paper。不多說,直接上題。
題目1:Two IB students carried out a project on the chemistry of bleach.
(b) Some of the group 17 elements, the halogens, show variable valency.
(i) Deduce the oxidation states of chlorine and iodine in the following species. [1]
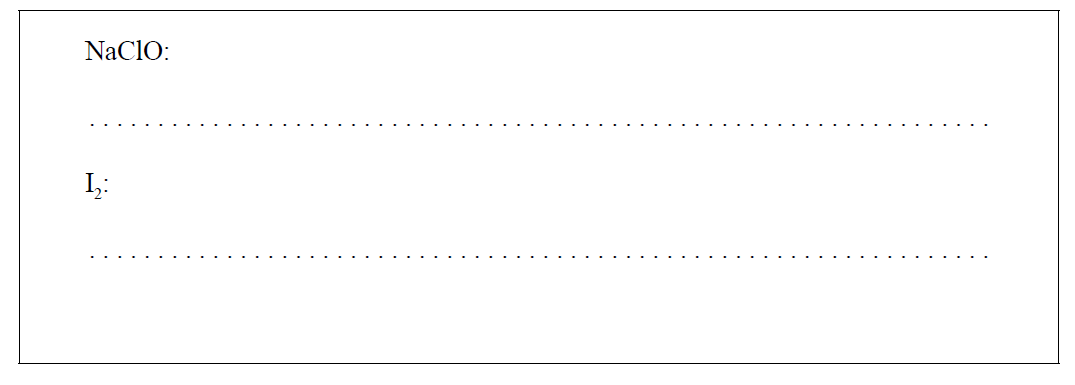
(ii) Deduce, with a reason, the oxidizing agent in the reaction of hypochlorite ions with iodide ions in part (a). [1]
(iii) From a health and safety perspective, suggest why it is not a good idea to use hydrochloric acid when acidifying the bleach. [1]
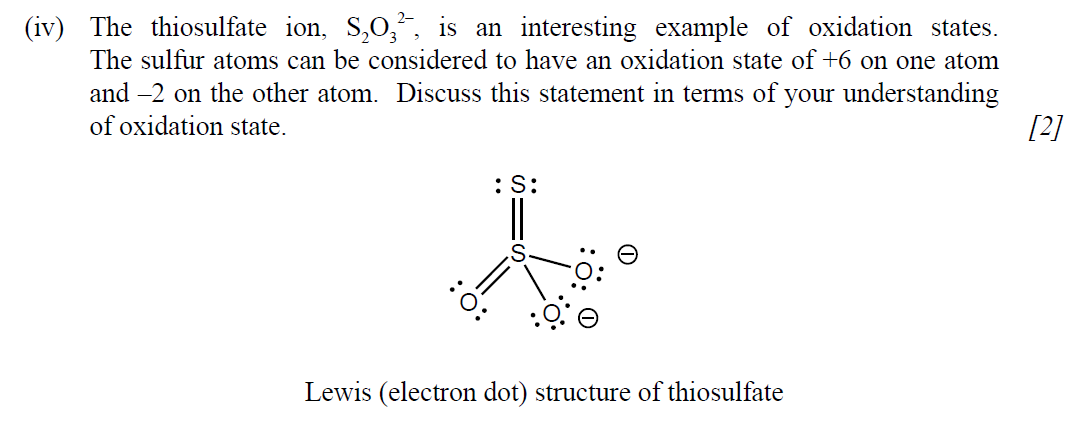
題目難度:簡單。
考點分析:考查group 17 elements的相關性質
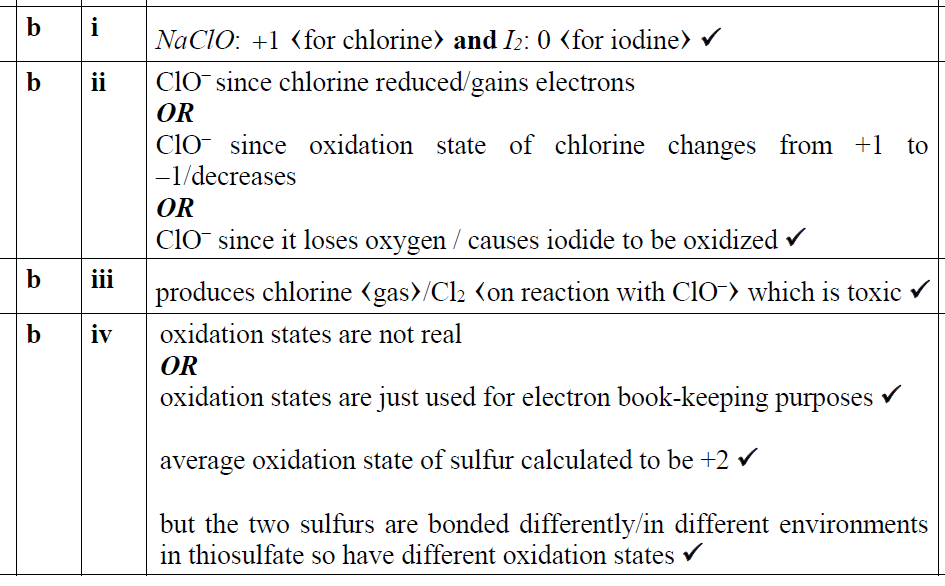
題目分析:第1問Cl是+1,I是-1;第2問oxidizing agent gains electrons,所以hypochlorite ions是oxidizing agent;第3問因為會產生有毒的氯氣;第4問參看下面答案就很容易理解了。
正確答案:
題目2:Two IB students carried out a project on the chemistry of bleach.
(i) State the condensed electron configuration of sulfur. [1]
(ii) Deduce the orbital diagram of sulfur, showing all the orbitals present in the diagram. [1]
題目難度:簡單。
考點分析:考查electron configuration和orbital diagram
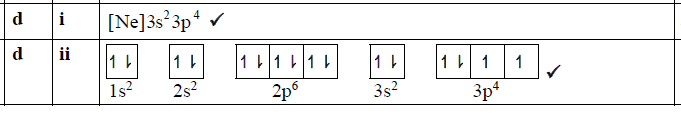
題目分析:S最外層有6個電子,其中3s有2個,3p有4個;3p軌道有3個,其中1個軌道有2個電子,另外2個軌道各1個電子。
正確答案:
掃碼添加翰林顧問老師,可一對一制定國際課程規劃
【免費領取】IBDP/IB 備考資料合集~


最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









