- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
Alevel有機化學一定要掌握的11個定義!
Hydrocarbon:
A compound consisting of hydrogen and carbon only
這個概念很好理解,只有H和O組成的化合物,比如CH4.
Saturated:
Carbon chains that contain the maximum number of hydrogens per carbon; only single C-C bonds
飽和鍵,化合物中C-C之間都是單鍵
Unsaturated:
Carbon chains that don’t have the maximum number of hydrogens per carbon; contain C=C double bonds不飽和鍵,C和C之間是雙鍵(Double bond)或者三鍵(Triple bond)
Empirical formula:
The simplest whole number ratio of atoms of each element in the compound
最簡式;比如C2H6的Emprical fomula是CH3
Molecular formula:
The actual number of each type of atom in the compound
分子式;明確的給出每一種原子的個數,比如C2H6O
General formula:
Algebraic formula for a homologous series
通式;表示同一類化合物分子組成的公式,比如alkane,烷烴類的通式為CnH2n+2
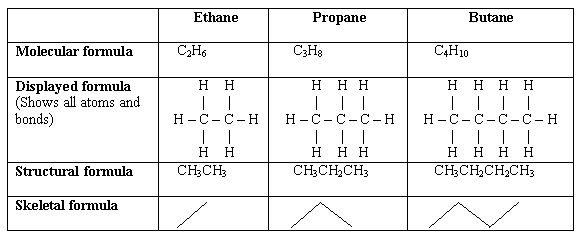
Structural formula:
Shows the minimal detail of the arrangement of atoms in a molecule結構式
Displayed formula:
Shows all the covalent bonds present in a molecule
展示所有的原子和鍵
Skeletal formula:
Shows the simplest organic formula of the carbon skeleton and associated functional groups
Homologous Series:
Families of organic compounds with the same functional group and general formula
同系物;是指結構相似、通式相同、分子組成相差若干個“CH2”原子團的有機化合物;是同一類物質(含有相同且數量相等的官能團。比如:CH4;C2H6;C3H8
Functional group:
The atom/group of atoms which provide the chemical properties of a substance
官能團,是決定有機化合物的化學性質的原子或原子團。常見官能團碳碳雙鍵、碳碳三鍵、羥基、羧基、醚鍵、醛基、羰基等。有機化學反應主要發生在官能團上,官能團對有機物的性質起決定作用,如—X、OH、-CHO、-COOH、-NO2、-SO3H、-NH2、RCO-。

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









