- 翰林提供學術活動、國際課程、科研項目一站式留學背景提升服務!
- 400 888 0080
「每日一練」ALevel化學例題解析及練習
每日一練ALevel Chemistry
今日知識點:Ionisation energy and valence electrons
例題
The table gives the successive ionisation energies for an element X.
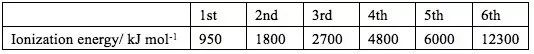
What could be the formula of the chloride of X
A?? XCl
B?? XCl2
C?? XCl3
D?? XCl4
解析
Answer: C
Definition of Ionisation Energy
A? →? A+ + e-
The energy required for the above reaction, or removing an electron. (挪走一個電子需要的能量)
Based on the table, there is a “jump” from the 5th to the 6th ionization energies, so there are 5 valence electrons.
5th 和6th 之間的數字變化比較大, 說明挪走第6個電子的需要很高能量,很困難,即挪走5個電子后達到8電子穩定結構,所以最外層有5個電子。
If X has 5 valence electrons, it needs to get an additional 3 electrons to form an Octect. Since each chlorine can provide 1 electron to form a bonding pair, 3 Cl is needed, forming XCl3.
氯化物中心的X需要形成8電子穩定結構,所以還需要額外和Cl共用3個電子,每個Cl只能共用1個電子,所以需要3個Cl.
下面我們為大家準備了一道同類型的題目,請大家一起來試試解答。
Your turn
Three successive elements in the Periodic Table have first ionization energies which have the pattern shown in the diagram.
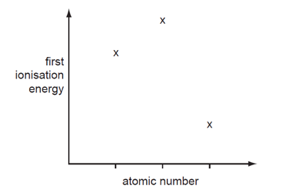
What could be the first element of the sequence?
A?? C
B?? N
C?? F
D?? Na
.
.
.
.
.
.
.
.
.
.
.
.
.
Correct Answer:C

最新發布
? 2025. All Rights Reserved. 滬ICP備2023009024號-1









